Metal-Organic Frameworks in Green Analytical Chemistry
Abstract
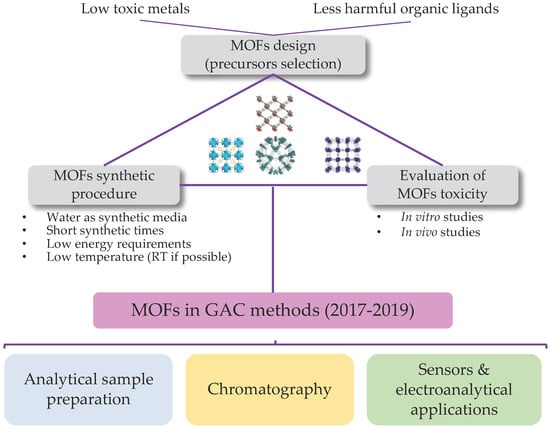
:1. Introduction
2. Green Considerations during MOFs Preparation
2.1. MOFs Design
2.2. MOFs Synthesis
2.3. Evaluation of MOFs Toxicity
3. Analytical Methods Incorporating MOFs
3.1. MOFs in Analytical Sample Preparation
3.2. MOFs in Chromatography
3.3. MOFs as Sensors in Spectroscopic and Alectroanalytical Methos
4. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matsuyama, S.; Munakata, M.; Emori, T.; Kitagawa, S. Synthesis and Crystal Structures of Novel One-dimensional - Polymers, [{M(bpen)X∞},1 [M = Cu’, X = PF6-; M = Ag’, X = ClO4-; bpen = trans-I,2-bis(2-pyridyl)ethylene] and [{Cu(bpen)(CO)(CH3CN)(PF6)}∞]. J. Chem. Soc. Dalton Trans. 1991, 1, 2869–2874. [Google Scholar]
- Yaghi, O.M.; Hailian, L. Hydrothermal Synthesis of a Metal-Organic Framework Containing Large Rectangular Channels. J. Am. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Riou, D.; Férey, G. Hybrid open frameworks (MIL-n). Part 3: Crystal structures of the HT and LT forms of MIL-7: A new vanadium propylenediphosphonate with an open-framework. Influence of the synthesis temperature on the oxidation state of vanadium within the same structural type. J. Mater. Chem. 1998, 8, 2733–2735. [Google Scholar]
- Farha, O.K.; Eryazici, I.; Jeong, N.C.; Hauser, B.G.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Nguyen, S.B.T.; Yazaydın, A.Ö.; Hupp, J.T. Metal−Organic Framework Materials with Ultrahigh Surface Areas: Is the Sky the Limit? J. Am. Chem. Soc. 2012, 134, 15016–15021. [Google Scholar] [CrossRef]
- Khan, N.A.; Hasan, Z.; Jhung, S.H. Adsorptive removal of hazardous materials using metal-organic frameworks (MOFs): A review. J. Hazard. Mater. 2013, 244–245, 444–456. [Google Scholar] [CrossRef]
- Moghadam, P.Z.; Li, A.; Wiggin, S.B.; Tao, A.; Maloney, A.G.P.; Wood, P.A.; Ward, S.C.; Fairen-Jimenez, D. Development of a Cambridge Structural Database Subset: A Collection of Metal–Organic Frameworks for Past, Present, and Future. Chem. Mater. 2017, 29, 2618–2625. [Google Scholar] [CrossRef]
- Stavila, V.; Talin, A.A.; Allendorf, M.D. MOF-based electronic and opto-electronic devices. Chem. Soc. Rev. 2014, 43, 5994–6010. [Google Scholar] [CrossRef] [Green Version]
- Bae, Y.S.; Snurr, R.Q. Development and evaluation of porous materials for carbon dioxide separation and capture. Angew. Chem. Int. Ed. 2011, 50, 11586–11596. [Google Scholar] [CrossRef]
- Li, H.; Wang, K.; Sun, Y.; Lollar, C.T.; Li, J.; Zhou, H.-C. Recent advances in gas storage and separation using metal–organic frameworks. Mater. Today 2018, 21, 108–121. [Google Scholar] [CrossRef]
- Ma, S.; Zhou, H.-C. Gas storage in porous metal–organic frameworks for clean energy applications. Chem. Commun. 2010, 46, 44–53. [Google Scholar] [CrossRef]
- Kang, Y.-S.; Lu, Y.; Chen, K.; Zhao, Y.; Wang, P.; Sun, W.Y. Metal–organic frameworks with catalytic centers: From synthesis to catalytic application. Coord. Chem. Rev. 2019, 378, 262–280. [Google Scholar] [CrossRef]
- Abánades-Lázaro, I.; Forgan, R.S. Application of zirconium MOFs in drug delivery and biomedicine. Coord. Chem. Rev. 2019, 380, 230–259. [Google Scholar] [CrossRef]
- Pacheco-Fernández, I.; González-Hernández, P.; Pasán, J.; Ayala, J.H.; Pino, V. The rise of metal-organic frameworks in analytical chemistry. In Handbook of Smart Materials in Analytical Chemistry; De la Guardia, M., Esteve-Turrillas, F.A., Eds.; Wiley: Hoboken, NJ, USA, 2016; Volume 1, pp. 463–502. [Google Scholar]
- Rocío-Bautista, P.; Pacheco-Fernández, I.; Pasán, J.; Pino, V. Are metal-organic frameworks able to provide a new generation of solid-phase microextraction coatings?—A review. Anal. Chim. Acta 2016, 939, 26–41. [Google Scholar] [CrossRef]
- Rocío-Bautista, P.; González-Hernández, P.; Pino, V.; Pasán, J.; Afonso, A.M. Metal-organic frameworks as novel sorbents in dispersive-based microextraction approaches. Trends Anal. Chem. 2017, 90, 114–134. [Google Scholar] [CrossRef]
- Maya, F.; Cabello, C.P.; Frizzarin, R.M.; Estela, J.M.; Palomino, G.T.; Cerdà, V. Magnetic solid-phase extraction using metal-organic frameworks (MOFs) and their derived carbons. Trends Anal. Chem. 2017, 90, 142–152. [Google Scholar] [CrossRef]
- Wang, X.; Ye, N. Recent advances in metal-organic frameworks and covalent organic frameworks for sample preparation and chromatographic analysis. Electrophoresis 2017, 38, 3059–3078. [Google Scholar] [CrossRef]
- Hashemi, B.; Zohrabi, P.; Raza, N.; Kim, K.-H. Metal-organic frameworks as advanced sorbents for the extraction and determination of pollutants from environmental, biological, and food media. Trends Anal. Chem. 2017, 97, 65–82. [Google Scholar] [CrossRef]
- Maya, F.; Cabello, C.P.; Figuerola, A.; Palomino, G.T.; Cerdà, V. Immobilization of Metal–Organic Frameworks on Supports for Sample Preparation and Chromatographic Separation. Chromatographia 2019, 82, 361–375. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Z. Metal-organic frameworks as stationary phase for application in chromatographic separation. J. Chromatogr. A 2017, 1530, 1–18. [Google Scholar] [CrossRef]
- Xiaoxin, L.; Lun, S.; Sha, C. Application of Metal-Organic Frameworks in Chromatographic Separation. Acta Chim. Sin. 2016, 74, 969–979. [Google Scholar]
- Li, A.; Liu, X.; Chai, H.; Huang, Y. Recent advances in the construction and analytical applications of metal-organic frameworks-based nanozymes. Trends Anal. Chem. 2018, 105, 391–403. [Google Scholar] [CrossRef]
- Feldblyum, J.I.; Keenan, E.A.; Matzger, A.J.; Maldonado, S. Photoresponse Characteristics of Archetypal Metal–Organic Frameworks. J. Phys. Chem. C 2012, 116, 3112–3121. [Google Scholar] [CrossRef]
- Yadav, D.K.; Ganesan, V.; Marken, F.; Gupta, R.; Sonkar, P.K. Metal@MOF Materials in Electroanalysis: Silver-Enhanced Oxidation Reactivity Towards Nitrophenols Adsorbed into a Zinc Metal Organic Framework—Ag@MOF-5(Zn). Electrochim. Acta 2016, 219, 482–491. [Google Scholar] [CrossRef]
- Filippou, O.; Bitas, D.; Samanidou, V. Green approaches in sample preparation of bioanalytical samples prior to chromatographic analysis. J. Chromatogr. B 2017, 1043, 44–62. [Google Scholar] [CrossRef]
- Sheldon, R.A. Fundamentals of green chemistry: Efficiency in reaction design. Chem. Soc. Rev. 2012, 41, 1437–1451. [Google Scholar] [CrossRef]
- Ludwig, J.K. Green Chemistry: An Introductory Text. Green Chem. Lett. 2017, 10, 30–31. [Google Scholar] [CrossRef] [Green Version]
- Valcárcel, M. Principles of Analytical Chemistry; Springer: Berlin, Germany, 2000; ISBN 978-3-642-57157-2. [Google Scholar]
- Koel, M.; Kaljurand, M. Green Analytical Chemistry; The Royal Society of Chemistry: Cambridge, UK, 2010; ISBN 978-1-84755-872-5. [Google Scholar]
- Namieśnik, J. Trends in Environmental Analytics and Monitoring. Crit. Rev. Anal. Chem. 2000, 30, 221–269. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998; p. 30. [Google Scholar]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Szczepańska, N.; de la Guardia, M.; Namieśnik, J. Miniaturized solid-phase extraction techniques. Trends Anal. Chem. 2015, 73, 19–38. [Google Scholar] [CrossRef]
- Tobiszewski, M.; Namieśnik, J. Greener organic solvents in analytical chemistry. Curr. Opin. Green Sustain. Chem. 2017, 5, 1–4. [Google Scholar] [CrossRef]
- Pacheco-Fernández, I.; Pino, V. Green solvents in analytical chemistry. Curr. Opin. Green Sust. Chem. 2019, 18, 42–50. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–715. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 12304441–123044412. [Google Scholar] [CrossRef]
- Bai, Y.; Dou, Y.; Xie, L.-H.; Rutledge, W.; Li, J.-R.; Zhou, H.-C. Zr-based metal–organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef]
- Rajkumar, T.; Kukkar, D.; Kim, K.-H.; Sohn, J.R.; Deed, A. Cyclodextrin-metal–organic framework (CD-MOF): From synthesis to applications. J. Ind. Eng. Chem. 2019, 72, 50–66. [Google Scholar] [CrossRef]
- Gaab, M.; Trukhan, N.; Maurer, S.; Gummaraju, R.; Müller, U. The progression of Al-based metal-organic frameworks—From academic research to industrial production and applications. Micropor. Mesopor. Mater. 2012, 157, 131–136. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.; Kim, J.; Lee, D. Metal–organic frameworks derived from zero-valent metal substrates: Mechanisms of formation and modulation of properties. Adv. Funct. Mater. 2019, 29, 1808466. [Google Scholar] [CrossRef]
- Sheta, S.M.; El-Sheikh, S.M.; Abd-Elzaher, M.M. A novel optical approach for determination of prolactin based on Pr-MOF nanofibers. Anal. Bioanal. Chem. 2019. [Google Scholar] [CrossRef]
- Reinsch, H. “Green” Synthesis of Metal-Organic Frameworks. Eur. J. Inorg. Chem. 2016, 27, 4290–4299. [Google Scholar] [CrossRef]
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.M.; Hill, M.R. New synthetic routes towards MOF production at scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef] [Green Version]
- Klimakow, M.; Klobes, P.; Thunemann, A.F.; Rademann, K.; Emmerling, F. Mechanochemical synthesis of Metal-Organic Frameworks: A fast and facile approach toward quantitative yields and high specific surface areas. Chem. Mater. 2010, 22, 5216–5221. [Google Scholar] [CrossRef]
- Joaristi, A.M.; Juan-Alcañiz, J.; Serra-Crespo, P.; Kapteijn, F.; Gascón, J. Electrochemical synthesis of some archetypical Zn2+, Cu2+, and Al3+ Metal Organic Frameworks. Cryst. Growth Des. 2012, 12, 3489–3498. [Google Scholar] [CrossRef]
- Julien, P.A.; Mottillo, C.; Friscic, T. Metal-Organic Frameworks meet scalable and sustainable synthesis. Green Chem. 2017, 19, 2729–2747. [Google Scholar] [CrossRef]
- Li, P.; Cheng, F.F.; Xiong, W.W.; Zhang, Q.C. New synthetic strategies to prepare metal-organic frameworks. Inorg. Chem. Front. 2018, 5, 2693–2708. [Google Scholar] [CrossRef]
- Khan, N.A.; Jhung, S.-H. Synthesis of Metal-Organic Frameworks (MOFs) with microwave or ultrasound: Rapid reaction, phase selectivity, and size reduction. Coord. Chem. Rev. 2015, 285, 11–23. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, M.; Getachew, N.; Díaz, K.; Díaz-García, M.; Chebude, Y.; Díaz, I. Synthesis of metal–organic frameworks in water at room temperature: Salts as linker sources. Green Chem. 2015, 17, 1500–1509. [Google Scholar] [CrossRef]
- Zhang, J.; Bu, J.T.; Chen, S.; Wu, T.; Zheng, S.; Chen, Y.; Nieto, R.A.; Feng, P.; Bu, X. Urothermal synthesis of crystalline porous materials. Angew. Chem. Int. Ed. 2010, 49, 8876–8879. [Google Scholar] [CrossRef]
- Tranchemontagne, D.J.; Hunt, J.R.; Yaghi, O.M. Room temperature synthesis of metal-organic frameworks: MOF-5, MOF-74, MOF-177, MOF-199, and IRMOF-0. Tetrahedron 2008, 64, 8553–8557. [Google Scholar] [CrossRef]
- Bayliss, P.A.; Ibarra, I.A.; Pérez, E.; Yang, S.; Tang, C.C.; Poliakoff, M.; Schröder, M. Synthesis of metal–organic frameworks by continuous flow. Green Chem. 2014, 16, 3796–3802. [Google Scholar] [CrossRef]
- Rocío-Bautista, P.; Pino, V.; Ayala, J.H.; Ruiz-Pérez, C.; Vallcorba, O.; Afonso, A.M.; Pasán, J. A green metal–organic framework to monitor water contaminants. RSC Adv. 2018, 8, 31304–31310. [Google Scholar] [CrossRef]
- Noh, H.; Kung, C.-W.; Islamoglu, T.; Peters, A.W.; Liao, Y.; Li, P.; Garibay, S.J.; Zhang, X.; DeStefano, M.R.; Hupp, J.T.; et al. Room Temperature Synthesis of an 8-Connected Zr-Based Metal–Organic Framework for Top-Down Nanoparticle Encapsulation. Chem. Mater. 2018, 30, 2193–2197. [Google Scholar] [CrossRef]
- Lenstra, D.C.; Rutjes, F.P.J.T. Organic synthesis in flow: Toward higher levels of sustainability. In Sustainable Flow Chemistry: Methods and Applications; Vaccaro, L., Ed.; Wiley: Hoboken, NJ, USA, 2017; pp. 103–134. [Google Scholar]
- Leardi, R. Experimental design in chemistry: A tutorial. Anal. Chim. Acta 2009, 652, 161–172. [Google Scholar] [CrossRef]
- Tamames-Tabar, C.; Cunha, D.; Imbuluzqueta, E.; Ragon, F.; Serre, C.; Blanco-Prieto, C.M.; Horcajada, P. Cytotoxicity of nanoscaled metal–organic frameworks. J. Mater. Chem. B 2014, 2, 262–271. [Google Scholar] [CrossRef]
- Chen, G.; Leng, X.; Luo, J.; You, L.; Qu, C.; Dong, X.; Huang, H.; Yin, X.; Ni, J. In Vitro Toxicity Study of a Porous Iron (III) Metal-Organic Framework. Molecules 2019, 24, 1211. [Google Scholar] [CrossRef]
- Horcajada, P.; Chalati, T.; Serre, C.; Gille, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtau, D.; Clayette, P.; Kreuz, C.; et al. Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–179. [Google Scholar] [CrossRef]
- Su, F.; Jia, Q.; Li, Z.; Wang, M.; He, L.; Peng, D.; Song, Y.; Zhang, Z.; Fang, S. Aptamer-templated silver nanoclusters embedded in zirconium metal–organic framework for targeted antitumor drug delivery. Micropor. Mesopor. Mater. 2019, 275, 152–162. [Google Scholar] [CrossRef]
- Vinogradov, V.V.; Drozdov, A.S.; Mingabudinova, L.R.; Shabanova, E.M.; Kolchina, N.O.; Anastasova, E.I.; Markova, A.A.; Shtil, A.A.; Milichko, V.A.; Starova, G.L.; et al. Composites based on heparin and MIL-101(Fe): The drug releasing depot for anticoagulant therapy and advanced medical nanofabrication. J. Mater. Chem. B 2018, 6, 2450–2459. [Google Scholar] [CrossRef]
- Ma, A.; Luo, Z.; Gu, C.; Li, B.; Liu, J. Cytotoxicity of a metal–organic framework: Drug delivery. Inorg. Chem. Commun. 2017, 77, 68–71. [Google Scholar] [CrossRef]
- Cheplakova, A.M.; Solovieva, A.O.; Pozmogova, T.N.; Vorotnikov, Y.A.; Brylev, K.A.; Vorotnikova, N.A.; Vorontsova, E.V.; Mironov, Y.V.; Poveshchenko, A.F.; Kovalenko, A.K.; et al. Nanosized mesoporous metal–organic framework MIL-101 as a nanocarrier for photoactive hexamolybdenum cluster compounds. J. Inorg. Biochem. 2017, 166, 100–107. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Y.; Liu, L.; Wan, W.; Guo, P.; Nyström, A.M.; Zou, X. One-pot Synthesis of Metal−Organic Frameworks with Encapsulated Target Molecules and Their Applications for Controlled Drug Delivery. J. Am. Chem. Soc. 2016, 138, 962–968. [Google Scholar] [CrossRef]
- Sifaoui, I.; López-Arencibia, A.; Martín-Navarro, C.M.; Reyes-Batlle, M.; Wagner, C.; Chiboub, O.; Mejri, M.; Valladares, B.; Abderrabba, M.; Piñero, J.E.; et al. Programmed cell death in Acanthamoeba castellanii Neff induced by several molecules present in olive leaf extracts. PLoS ONE 2017, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Lei, D.; Duan, X.; Zhang, S.; Song, W.; Hou, C.; Tang, R. Design and preparation of metal-organic framework papers with enhanced mechanical properties and good antibacterial capacity. Carbohydr. Polym. 2018, 192, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, L.; Yang, Z.; Liu, P.; Wang, J.; Cao, J.; Shen, A.; Xu, Z.; Wang, J. An efficient tumor-inducible nanotheranostics for magnetic resonance imaging and enhanced photodynamic therapy. Chem. Eng. J. 2019, 358, 969–979. [Google Scholar] [CrossRef]
- Xin, X.-T.; Cheng, J.-Z. A mixed-ligand approach for building a N-rich porous metal-organic framework for drug release and anticancer activity against oral squamous cell carcinoma. J. Coord. Chem. 2018, 71, 3565–3574. [Google Scholar] [CrossRef]
- Lázaro, I.A.; Haddad, S.; Rodrigo-Muñoz, J.M.; Marshall, R.J.; Sastre, B.; del Pozo, V.; Fairen-Jimenez, D.; Forgan, R.S. Surface-Functionalization of Zr-Fumarate MOF for Selective Cytotoxicity and Immune System Compatibility in Nanoscale Drug Delivery. ACS Appl. Mater. Interf. 2018, 10, 31146–31157. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Li, L.; Li, S.; Bai, Y.; Liu, H. Metal–organic frameworks induce autophagy in mouse embryonic fibroblast cells. Nanoscale 2018, 10, 18161–18168. [Google Scholar] [CrossRef] [PubMed]
- Armenta, S.; Garrigues, S.; Esteve-Turrillas, F.A.; Guardia, M. Green extraction techniques in green analytical chemistry. Trends Anal. Chem. 2019. [Google Scholar] [CrossRef]
- Pacheco-Fernández, I.; González-Hernández, P.; Rocío-Bautista, P.; Trujillo-Rodríguez, M.J.; Pino, V. Main uses of Microwaves and Ultrasounds in Analytical Extraction Schemes: An Overview. In Analytical Separation Science; Anderson, J.L., Stalcup, A., Berthod, A., Pino, V., Eds.; Wiley: Hoboken, NJ, USA, 2016; Volume 5, pp. 1469–1501. [Google Scholar]
- Yamini, Y.; Rezazadeh, M.; Seidi, S. Liquid-phase microextraction—The different principles and configurations. Trends Anal. Chem. 2019, 112, 264–272. [Google Scholar] [CrossRef]
- Mogaddam, M.R.A.; Mohebbi, A.; Pazhohan, A.; Khodadadeian, F.; Farajzadeh, M.A. Headspace mode of liquid phase microextraction: A review. Trends Anal. Chem. 2019, 110, 8–14. [Google Scholar] [CrossRef]
- Chisvert, A.; Cárdenas, S.; Lucena, R. Dispersive micro-solid phase extraction. Trends Anal. Chem. 2019, 112, 224–247. [Google Scholar] [CrossRef]
- Olcer, Y.A.; Tascon, M.; Eroglu, A.E.; Boyacı, E. Thin film microextraction: Towards faster and more sensitive microextraction. Trends Anal. Chem. 2019, 113, 97–101. [Google Scholar] [CrossRef]
- Llompart, M.; Celeiro, M.; García-Jares, C.; Dagnac, T. Environmental applications of solid-phase microextraction. Trends Anal. Chem. 2019, 112, 224–247. [Google Scholar] [CrossRef]
- Gao, G.; Xing, X.; Liu, T.; Wang, J.; Hou, X. UiO-66(Zr) as sorbent for porous membrane protected micro-solid-phase extraction androgens and progestogens in environmental water samples coupled with LC-MS/MS analysis: The application of experimental and molecular simulation method. Microchem. J. 2019, 146, 126–133. [Google Scholar] [CrossRef]
- Kahkha, M.R.R.; Daliran, S.; Oveisi, A.R.; Kaykhaii, M.; Sepehri, Z. The Mesoporous Porphyrinic Zirconium Metal-Organic Framework for Pipette-Tip Solid-Phase Extraction of Mercury from Fish Samples Followed by Cold Vapor Atomic Absorption Spectrometric Determination. Food Anal. Methods. 2017, 10, 2175–2184. [Google Scholar] [CrossRef]
- Gao, G.; Li, S.; Li, S.; Wang, Y.; Zhao, P.; Zhang, X.; Hou, X. A combination of computational−experimental study on metal-organic frameworks MIL-53(Al) as sorbent for simultaneous determination of estrogens and glucocorticoids in water and urine samples by dispersive micro-solid-phase extraction coupled to UPLC-MS/MS. Talanta 2018, 180, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Taima-Mancera, I.; Rocío-Bautista, P.; Pasán, J.; Ayala, J.H.; Ruiz-Pérez, C.; Afonso, A.M.; Lago, A.B.; Pino, V. Influence of ligand functionalization of UiO-66-based Metal-Organic Frameworks when used as sorbents in dispersive solid-phase analytical microextraction for different aqueous organic pollutants. Molecules 2018, 23, 2869. [Google Scholar] [CrossRef]
- Kashanaki, R.; Ebrahimzadeh, H.; Moradi, M. Metal–organic framework based micro solid phase extraction coupled with supramolecular solvent microextraction to determine copper in water and food samples. New J. Chem. 2018, 42, 5806–5813. [Google Scholar] [CrossRef]
- González-Hernández, P.; Lago, A.B.; Pasán, J.; Ruiz-Pérez, C.; Ayala, J.H.; Afonso, A.M.; Pino, V. Application of a pillared-layer Zn-triazolate metal-organic framework in the dispersive miniaturized solid-phase extraction of personal care products from wastewater samples. Molecules 2019, 24, 690. [Google Scholar] [CrossRef]
- Boontongto, T.; Siriwong, K.; Burakham, R. Amine-Functionalized Metal–Organic Framework as a New Sorbent for Vortex-Assisted Dispersive Micro-Solid Phase Extraction of Phenol Residues in Water Samples Prior to HPLC Analysis: Experimental and Computational Studies. Chromatographia 2018, 81, 735–747. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Liu, Q.-H.; Li, K.; Li, Y.; Chang, N. Magnetic iron(III)-based framework composites for the magnetic solid-phase extraction of fungicides from environmental water samples. J. Sep. Sci. 2018, 41, 1129–1137. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, B.; Yan, Y.; Liang, H.; Wu, D. Silica Protection–Sacrifice Functionalization of Magnetic Graphene with a Metal–Organic Framework (ZIF-8) to Provide a Solid-Phase Extraction Composite for Recognization of Phthalate Easers from Human Plasma Samples. Chromatographia 2019, 82, 625–634. [Google Scholar] [CrossRef]
- Liu, G.; Huang, X.; Lu, M.; Li, L.; Li, T.; Xu, D. Facile synthesis of magnetic zinc metal-organic framework for extraction of nitrogen-containing heterocyclic fungicides from lettuce vegetable samples. J. Sep. Sci. 2019, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, R.; Li, D.; Sun, P.; Su, P.; Yang, Y. Preparation of iron-based MIL-101 functionalized polydopamine@Fe3O4 magnetic composites for extracting sulfonylurea herbicides from environmental water and vegetable samples. J. Sep. Sci. 2018, 41, 2046–2055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yao, W.; Fu, D.; Zhang, C.; Zhao, H. Fabrication of magnetic zinc adeninate metal–organic frameworks for the extraction of benzodiazepines from urine and wastewater. J. Sep. Sci. 2018, 41, 1864–1870. [Google Scholar] [CrossRef] [PubMed]
- Safari, M.; Shahlaei, M.; Yamini, Y.; Shakorian, M.; Arkan, E. Magnetic framework composite as sorbent for magnetic solid phase extraction coupled with high performance liquid chromatography for simultaneous extraction and determination of tricyclic antidepressants. Anal. Chim. Acta 2018, 1034, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Lin, X.; Li, T.; Pang, T.; Dong, Y.; Zhuo, R.; Wang, Q.; Cao, Y.; Gan, N. A solid phase microextraction Arrow with zirconium metal–organic framework/molybdenum disulfide coating coupled with gas chromatography–mass spectrometer for the determination of polycyclic aromatic hydrocarbons in fish samples. J. Chromatogr. A 2019, 1592, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, J.; Guo, C.; Li, Y. Metal azolate framework-66-coated fiber for headspace solid-phase microextraction of polycyclic aromatic hydrocarbons. J. Chromatogr. A 2019, 1592, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Zhao, X.; Jin, Y.; Yang, G.; Li, Z.; Wang, J.; Zhao, R.; Li, Z. Determination of aromatic amines in the urine of smokers using a porous organic framework (JUC-Z2)-coated solid-phase microextraction fiber. J. Chromatogr. A 2018, 1555, 37–44. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, G.; Li, L.; Wang, X.; Li, N.; Zhao, R.-S.; Lin, J. Facile fabrication of MIL-96 as coating fiber for solid-phase microextraction of trihalomethanes and halonitromethanes in water samples. Chem. Eng. J. 2018, 350, 240–247. [Google Scholar] [CrossRef]
- Tian, Y.; Sun, M.; Wang, X.; Luo, C.; Feng, J. A Nanospherical Metal–Organic Framework UiO-66 for Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons. Chromatographia 2018, 81, 1053–1061. [Google Scholar] [CrossRef]
- Yang, J.-H.; Cui, C.-X.; Qu, L.-B.; Chen, J.; Zhou, X.-M.; Zhang, Y.-P. Preparation of a monolithic magnetic stir bar for the determination of sulfonylurea herbicides coupled with HPLC. Microchem. J. 2018, 141, 369–376. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, W.; Liao, X.; Wang, X.; Chen, Z. Covalent immobilization of metal organic frameworks onto chemical resistant poly(ether ether ketone) jacket for stir bar extraction. Anal. Chim. Acta 2018, 1025, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Ghani, M.; Ghoreishi, S.M.; Azamati, M. In-situ growth of zeolitic imidazole framework-67 on nanoporousanodized aluminum bar as stir-bar sorptive extraction sorbent fordetermining caffeine. J. Chromatogr. A 2018, 1577, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Khan, M.I.; Li, X.; Zhu, Q.-L.; Wu, X.-T. Recent Progress in Asymmetric Catalysis and Chromatographic Separation by Chiral Metal–Organic Frameworks. Catalysts 2018, 8, 120–148. [Google Scholar] [CrossRef]
- Li, X.; Li, B.; Liu, M.; Zhou, Y.; Zhang, L.; Qiao, X. Core−Shell Metal−Organic Frameworks as the Mixed-Mode Stationary Phase for Hydrophilic Interaction/Reversed-Phase Chromatography. ACS Appl. Mater. Interf. 2019, 11, 10320–10327. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cejuela, H.M.; Carrasco-Correa, E.J.; Shahat, A.; Simó-Alfonso, E.F.; Herrero-Martínez, J.M. Incorporation of metal-organic framework amino-modified MIL-101 into glycidyl methacrylate monoliths for nano LC Separation. J. Sep. Sci. 2019, 42, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, L.; Zhang, W. Preparation and evaluation of open-tubular capillary column combining a metal–organic framework and a brush-shaped polymer for liquid chromatography. J. Sep. Sci. 2018, 41, 2347–2353. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Huang, M.; Zhang, Z.; Yang, Q.; Su, B.; Yang, Y.; Ren, Q.; Bao, Z. Hybridization of metal–organic framework and monodisperse spherical silica for chromatographic separation of xylene isomers. Chin. J. Chem. Eng. 2019, 27, 818–826. [Google Scholar] [CrossRef]
- Ehrling, S.; Kutzscher, C.; Freund, P.; Müller, P.; Senkovska, I.; Kaskel, S. MOF@SiO2 core-shell composites as stationary phase in high performance liquid chromatography. Micropor. Mesopor. Mat. 2018, 263, 268–274. [Google Scholar] [CrossRef]
- Xu, X.; Wang, C.; Li, H.; Li, X.; Liu, B.; Singh, V.; Wang, S.; Sun, L.; Gref, R.; Zhan, J. Evaluation of drug loading capabilities of γ-cyclodextrin-metal organic frameworks by high performance liquid chromatography. J. Chromatogr. A 2017, 1488, 37–44. [Google Scholar] [CrossRef]
- Qu, Q.; Xuan, H.; Zhang, K.; Chen, X.; Ding, Y.; Feng, S.; Xu, Q. Core-shell silica particles with dendritic pore channels impregnated with zeolite imidazolate framework-8 for high performance liquid chromatography separation. J. Chromatogr. A 2017, 1505, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, L.; Zhang, J.-H.; He, Y.-J.; Li, Q.; Luo, L.; Zhang, M.; Yuan, L.-M. Homochiral metal-organic framework immobilized on silica gel by the interfacial polymerization for HPLC enantioseparations. J. Liq. Chromatogr. Relat. Technol. 2018, 1, 1–7. [Google Scholar] [CrossRef]
- Lang, L.; Shengming, X.; Junhui, Z.; Ling, C.; Pengjing, Z.; Liming, Y. A Gas Chromatographic Stationary of Homochiral Metal-peptide Framework Material and Its Applications. Chem. Res. Chin. Univ. 2017, 33, 24–30. [Google Scholar]
- Zheng, D.-D.; Wang, L.; Yang, T.; Zhang, Y.; Wang, Q.; Kurmoo, M.; Zeng, M.-H. A Porous Metal−Organic Framework [Zn2(bdc)(L-lac)] as a Coating Material for Capillary Columns of Gas Chromatography. Inorg. Chem. 2017, 56, 11043–11049. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.-D.; Zhang, Y.; Wang, L.; Kurmoo, M.; Zeng, M.-H. A rod-spacer mixed ligands MOF [Mn3(HCOO)2(Dcam)2(DMF)2]n as coating material for gas chromatography capillary column. Inorg. Chem. Com. 2017, 82, 34–38. [Google Scholar] [CrossRef]
- Tang, P.; Wang, R.; Chen, Z. In situ growth of Zr-based metal-organic framework UiO-66-NH2 for open-tubular capillary electrochromatography. Electrophoresis 2018, 39, 2619–2625. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mao, Z.; Chen, Z. In-situ growth of a metal organic framework composed of zinc(II), adeninate and biphenyldicarboxylate as a stationary phase for open-tubular capillary electrochromatography. Microchim. Acta 2019, 186, 53–61. [Google Scholar] [CrossRef]
- Wang, X.; Ye, N.; Hu, X.; Liu, Q.; Li, J.; Peng, L.; Ma, X. Open-tubular capillary electrochromatographic determination of ten sulfonamides in tap water and milk by a metal-organic framework-coated capillary column. Electrophoresis 2018, 39, 2236–2245. [Google Scholar] [CrossRef]
- Pan, C.; Lv, W.; Niu, X.; Wang, G.; Chen, H.; Chen, X. Homochiral zeolite-like metal-organic framework with DNA like double-helicity structure as stationary phase for capillary electrochromatography enantioseparation. J. Chromatogr. A 2018, 1541, 31–38. [Google Scholar] [CrossRef]
- Tachikawa, T.; Choi, J.R.; Fujitsuka, M.; Majima, T. Photoinduced charge-transfer processes on MOF-5 nanoparticles: Elucidating differences between metal-organic frameworks and semiconductor metal oxides. J. Phys. Chem. C 2008, 112, 14090–14101. [Google Scholar] [CrossRef]
- Ji, L.; Jin, Y.; Wu, K.; Wan, C.; Yang, N.; Tang, Y. Morphology-dependent electrochemical sensing performance of metal (Ni, Co, Zn)-organic frameworks. Anal. Chim. Acta 2018, 1031, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-S.; Dong, S.-J.; Wu, K.; Zhang, X.-L.; Jiang, J.; Yuan, J.; Zheng, M.-D. An ultrastable magnesium-organic framework as multi-responsive luminescent sensor for detecting trinitrotoluene and metal ions with high selectivity and sensitivity. Sens. Actuat. B 2019, 283, 255–261. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, Q.; Hou, B.; Cheng, Q.; Zhang, G. A Novel Magnesium Metal−Organic Framework as a Multiresponsive Luminescent Sensor for Fe(III) Ions, Pesticides, and Antibiotics with High Selectivity and Sensitivity. Inorg. Chem. 2018, 57, 13330–13340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yao, H.; Zhao, Y.; Li, X.; Zhang, G.; Yang, Y. Mixed matrix membranes incorporated with Ln-MOF for selective and sensitive detection of nitrofuran antibiotics based on inner filter effect. Talanta 2017, 174, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Asadi, F.; Azizi, S.N.; Ghasemi, S. Preparation of Ag nanoparticles on nano cobalt-based metal organic framework (ZIF-67) as catalyst support for electrochemical determination of hydrazine. J. Mater. Sci. Mater. Electron. 2019, 30, 5410–5420. [Google Scholar] [CrossRef]
- Peng, M.; Guan, G.; Deng, H.; Han, B.; Tian, C.; Zhuang, J.; Xu, Y.; Liu, W.; Lin, Z. PCN-224/rGO nanocomposite based photoelectrochemical sensor with intrinsic recognition ability for efficient p-arsanilic acid detection. Environ. Nano 2019, 6, 207–215. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, C.; Li, X.; Zhu, X.; Ye, B.; Xu, M. A sensitive aptasensor for the detection of b-amyloid oligomers based on metal–organic frameworks as electrochemical signal probes. Anal. Methods 2018, 10, 4430–4437. [Google Scholar] [CrossRef]
- Sachdeva, S.; Koper, S.J.H.; Sabetghadam, A.; Soccol, D.; Gravesteijn, D.J.; Kapteijn, F.; Sudhölter, E.J.R.; Gascon, J.; de Smet, L.C.P.M. Gas Phase Sensing of Alcohols by Metal Organic Framework−Polymer Composite Materials. ACS Appl. Mater. Interf. 2017, 9, 24926–24935. [Google Scholar] [CrossRef]
- Zhou, N.; Ma, Y.; Hu, B.; He, L.; Wang, S.; Zhang, Z.; Luc, S. Construction of Ce-MOF@COF hybrid nanostructure: Label-free aptasensor for the ultrasensitive detection of oxytetracycline residues in aqueous solution environments. Biosens. Bioelectron. 2019, 127, 92–100. [Google Scholar] [CrossRef]
- Li, J.; Xia, J.; Zhang, F.; Wang, Z.; Liu, Q. An electrochemical sensor based on copper-based metal-organic frameworks-graphene composites for determination of dihydroxybenzene isomers in water. Talanta 2018, 181, 80–86. [Google Scholar] [CrossRef]
- Das, A.; Biswas, S. A multi-responsive carbazole-functionalized Zr(IV)-based metal-organic framework for selective sensing of Fe(III), cyanide and p-nitrophenol. Sens. Actuat. B 2017, 250, 121–131. [Google Scholar] [CrossRef]
- Guo, X.-Y.; Dong, Z.-P.; Zhao, F.; Liu, Z.-L.; Wang, Y.-Q. Zinc(II)–organic framework as a multi-responsive photoluminescence sensor for efficient and recyclable detection of pesticide 2,6-dichloro- 4-nitroaniline, Fe(III) and Cr(VI). New J. Chem. 2019, 43, 2353–2361. [Google Scholar] [CrossRef]



| MOF (metallic ion & ligand) | Cytotoxicity Test Type | Cell Line Type | Toxicity Value (mg·L−1) | Ref. | |
|---|---|---|---|---|---|
| UiO-66 (Zr4+ & H2bdc 1) | MTT 2 | MCF-7 | >80 mg·L−1 | [61] | |
| MIL-100(Fe) (Fe3+ & H3btc 3) UiO-66 (Zr4+ & H2bdc 1) ZIF-8 (Zn2+ & 2-MIm 4) among many others | MTT 2 | HeLa & J774.2 HeLa & J774.2 HeLa & J774.2 | 1100 & 700 mg·L−1 400 & 60 mg·L−1 100 & 25 mg·L−1 | [58] | |
| [Zn6(L)3(DMA)4]·5DMA (Zn2+ & H4L 5) | MTT 2 | Hela & HEK293 | 5000 mg·L−1 | [63] | |
| MIL-101(Cr) (Cr3+ & H2bdc 1) | MTT 2 | Hep-2 | 744 mg·L−1 | [64] | |
| ZIF-8 (Zn2+ & 2-MIm 4) | MTT 2 | MCF-7 MDA-MB-231 MDA-MB-468 | 200 mg·L−1 400 mg·L−1 400 mg·L−1 | [65] | |
| NanoMOF based on Fe (Fe3+ & TCPP 6) | MTT 2 | 4T1 | >200 mg·L−1 | [68] | |
| MIL-100(Fe) (Fe3+ & H3btc 3) | MTT 2 | HL-7702 & HepG2 | 80 mg·L−1 | [59] | |
| MIL-88A (Fe3+ & fumaric acid) | MTT 2 | J774.A1 | 57 mg·L−1 | [60] | |
| [Zn(H3btc 3)(HME 7)] (DMAc 8)(H2O) (Zn2+ & H3btc 3 & HME 7) | MTT 2 | SCC-251 & HSC-4 | 100 mg·L−1 | [69] | |
| UiO-64 (Zr4+ & fumaric acid) | MTT 2 | J774 & PBLs | 200 mg·L−1 | [70] | |
| CIM-80 (Al3+ & mesaconic acid) | alamarBlue® 9 | J774.1 | >2000 mg·L−1 | [54] | |
| ZIF-67 (Co2+ & 2-MIm 4) ZIF-8 (Zn2+ & 2-MIm 4) | CCK-8 10 | 293T | >80 mg·L−1 20 mg·L−1 | [67] | |
| 1 terephthalic acid (benzene-1,4-dicarboxylic acid) 2 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide dye 3 trimesic acid (benzene-1,3,5-tricarboxylic acid) 4 2-methylimidazole 5 [1,1’:3’,1’’-terphenyl]-3,3’’,5,5’’-tetracarboxylic acid 6 tetrakis(4-carboxyphenyl)porphyrin | 7 protonated melamine 8 N,N-dimethylacetamide 9 from resazurin (blue) to resorufin dye (fluorescent red-pink) 10 cell-counting kit assay, from WST-8 (colorless) to WST-8 formazan dye (orange) | ||||
| MOF (metallic ion & ligand) Synthetic Solvent (mL)/T (°C)/time (h) | Microextraction Format/Extraction Time (min) | MOF Amount | Analytes (number)/ Sample (amount) | LOD 1 (ng·L−1) /RSD 2 (%) | Analytical Technique | Ref. | |
|---|---|---|---|---|---|---|---|
| µ-SPE | |||||||
| UiO-66 (Zr4+ & H2bdc 3) DMF 4 (40)/120/24 | PP 5 cartridge (packing the MOF suspension)/40 | 10 mg | hormones (4)/waters (20 mL) | 2.0–10/ <6.5 | LC 6-MS/ MS 7 | [79] | |
| PCN-222 (Zr4+ & H2TCPP 8) DMF 4 (40)/100/1 | pipette tip (packing the MOF powder)/ <7 | 2 mg | Hg2+ (1)/fish (100 mL aqueous extract) | 20/8 | CVAAS 9 | [80] | |
| µ-dSPE | |||||||
| MIL-53(Al) (Al3+ & H2bdc 3) water (-)/210/72 | MOF powder/30 | 8 mg | hormones (8)/water & urine (8 mL) | 1.5–1000/<7.8 | LC 6-MS/ MS 7 | [81] | |
| UiO-66-NO2 (Zr4+ & O2N-H2bdc 10) DMF 4 (15)/150/24 | MOF powder/3 | 20 mg | EDCs 11 (9)/waters (20 mL) | 1.5–90/ <14 | LC 6-DAD 12 | [82] | |
| Al-Fu nano-flakes (Al3+ & fumaric acid) water (200)/90/0.5 | MOF powder/ 20 s | 30 mg | Cu2+ (1)/waters & food (25 mL sample or aqueous extract) | 0.2 µg·L−1/ 6.5 | GFAAS 13 | [83] | |
| CIM-81 (Zn2+ & Htz 14 + H2bdc 3) DMA 15 (15)/120/72 | MOF powder/1 | 10 mg | PCPs 16 (9)/waters (10 mL) | 0.5–1.5 µg·L−1/ <13 | LC 6-UV 17 | [84] | |
| CIM-80 (Al3+ & mesaconic acid) water (15)/150/3 | MOF powder/3 | 20 mg | PAHs 18 (15) & EDCs 11 (7)/waters (10 mL) | 0.75–9.3 (PAHs) & 0.11–21 µg·L−1 (EDCs)/ <19 | LC 6-UV 17 & LC 6-FD 19 | [54] | |
| H2N-MIL-53(Al) (Al3+ & H2N-H2bdc 20) DMF 4 (30)/130/72 | MOF powder/10 s | 30 mg | phenols (8)/waters (10 mL) | 0.4–13.3 µg·L−1/ <6.30 | LC 6-PDA 21 | [85] | |
| m-µ-dSPE | |||||||
| Fe3O4- CO2H@MIL-101-NH2 (Fe3+ & NH2-H2bdc 20) DMF 4 (15)/110/24 | heterogeneous composite powder/20 | 20 mg | fungicides (4)/waters (30) | 0.04–0.4 µg·L−1/ <10.2 | LC 6-UV 17 | [86] | |
| magG 22@ZIF-8 (Zn2+ & 2-MIm 23) methanol (60)/RT 24/24 | heterogeneous composite powder/10 | 10 mg | PAEs 25 (9)/diluted human plasma (1:3, overall volume ~9 mL) | 3–10/< 6.5 | GC 26-MS 7 | [87] | |
| Fe3O4/ GO 27-IRMOF-3 (Zn2+ & NH2-H2bdc 20) DMF 4 (40)/RT 24/3 | heterogeneous composite powder/30 | 10 mg | fungicides (5)/lettuce (10 mL aqueous extract) | 0.25–1.0 µg·L−1/ <7.3 | LC 6-MS/ MS 7 | [88] | |
| Fe3O4@PDA 28@ MIL-101(Fe) (Fe3+ & H2bdc 3) DMF 4 (80)/110/24 | heterogeneous composite powder/3 | 60 mg | SUHs 29 (4)/waters & vegetables (25 mL sample or aqueous extract) | 0.12–0.34 µg·L−1/ <4.8 | LC 6-PDA 21 | [89] | |
| Fe3O4-NH2/ bio-MOF-1 (Zn2+ & adenine) DMF 4 (67.5)/130/24 | heterogeneous composite powder/40 | 15 mg | BZPs 30 (6)/ urine & waters (40 mL) | 0.71–2.49/<8.8 | LC 6-MS 7 | [90] | |
| Fe3O4@ TMU-10 (Co2+ & H2oba 31) DMF 4 (10)/145/48 | heterogeneous composite powder/4 | 5 mg | TCAs 32 (2)/plasma & urine (6 mL) | 2–4 µg·L−1/ <5.2 | LC 6-UV 17 | [91] | |
| SPME | |||||||
| UiO-66/MoS2 (Zr4+ & H2bdc 3 + MoS2) DMF 4 (20)/120/24 | stainless steel arrow (MOF attached with epoxy glue)/30 | - × 25 µm thickness | PAHs 18 (16)/fish (10 mL alkaline extract) | 0.11–1.40/<8.6 | HS-SPME 33- GC 26-MS 7 | [92] | |
| MAF-66 (Zn2+ & H2N-Htz 34) isopropanol (50)/RT 24/72 | stainless steel fiber (layer-by-layer deposition)/40 | 3.0 cm × 15 µm thickness | PAHs 18 (7)/water, potatoes & roast pork (10 mL sample or aqueous extract) | 0.1–7.5/ <4.2 | HS-SPME 33-GC 26-FID 35 | [93] | |
| JUC-Z2 (Ni2+ & 2,2’-bipyridyl) DMF 4 (80)/80/1 | functionalized fused silica fiber (in-situ sol-gel method)/40 | - × 80 µm thickness | aromatic amines (2)/urine (10 mL) | 0.010–0.012/ <7.7 | HS-SPME 33- GC 26-MS/MS 7 | [94] | |
| MIL-96 (Al3+ & H3btc 36) water (30)/200/24 | stainless steel fiber (MOF attached with epoxy glue)/30 | 2.0 cm × 80 µm thickness | THMs 37 (4) and TCNM 38/waters (10 mL) | 3.0–11/ <10.1 | HS-SPME 33- GC 26-MS 7 | [95] | |
| UiO-66 (Zr4+ & H2bdc 3) DMF 4 (40)/120/24 | stainless steel fiber (MOF attached with silicone sealant)/40 | 4.0 cm × 8.5 µm thickness | PAHs 18 (9)/waters (10 mL) | 10–30/ <5.6 | HS-SPME 33-GC 26-FID 35 | [96] | |
| SBSME | |||||||
| UiO-66-NH2 (Zr4+ & NH2-H2bdc 20) DMF 4 (10) & acetic acid (7)/120/24 | coated glass bar (in-situ polymerization method)/30 | 1 cm × 4000 µm thickness | SUHs 29 (5)/water & soil (5 mL sample or aqueous extract) | 40–840/ <13.8 | LC 6-UV 17 | [97] | |
| MIL-68@PEEK 39 (Al3+ & H2bdc 3) DMF 4 (10)/130/18.5 | coated dumbbell-shaped bar (covalent immobilization)/120 | 3 cm × 18.4 µm thickness | parabens (3)/creams & rabbit plasma (20 mL aqueous extract) | 1–2/ <9.74 | LC 6-MS 7 | [98] | |
| ZIF-67 (Co2+ & 2-MIm 23) methanol (80)/120/5 | anodized aluminum bar (in-situ growth)/20 | 1 cm × 500 µm thickness | caffeine (1)/beverage & urine (10 mL) | 50–100/ <6.1 | LC 6-UV 17 | [99] | |
| 1 limit of detection 2 inter-day relative standard deviation 3 terephthalic acid (benzene-1,4-dicarboxylic acid) 4 N,N-dimethylformamide 5 polypropylene 6 liquid chromatography 7 mass spectrometry 8 meso-tetra(4-carboxyphenyl)porphyrin 9 cold vapor atomic absorption spectroscopy 10 2-nitroterephthalic acid (2-nitrobenzene-1,4-dicarboxylic acid) 11 endocrine disrupting chemicals 12 diode array detector 13 graphite furnace atomic absorption spectroscopy 14 1,2,4-triazole 15 N,N-dimethylacetamide 16 personal care products 17 ultraviolet detector 18 polycyclic aromatic hydrocarbons 19 fluorescence detector 20 2-aminoterephthalic acid (2-aminobenzene-1,4-dicarboxylic acid) | 21 photodiode array detector 22 magnetic graphene 23 2-methylimidazole 24 room temperature 25 phthalate esters 26 gas chromatography 27 graphene oxide 28 polydopamine 29 sulfonylurea herbicides 30 benzodiazepines 31 4,4’-oxybis(benzoic acid) 32 tricyclic antidepressants 33 headspace solid-phase microextraction 34 3-amino-1,2,4-triazole 35 flame ionization detector 36 trimesic acid (benzene-1,3,5-tricarboxylic acid) 37 trihalomethanes 38 trichloronitromethane 39 polyether ether ketone -: non-reported | ||||||
| MOF (metallic ion & ligand) Synthetic solvent (mL)/T (°C)/time (h) | Stationary phase (type)/Type of column | Size & N 1 (plates·m−1)/ k 2/α 3 | Detector | Analytes (number) | Ref. |
|---|---|---|---|---|---|
| LC | |||||
| UiO-67 (Zr4+ & H2bdpc 4) DMF 5 (40) & acetic acid (6)/120/24 | UiO-67@SiO2 (core-shell composite)/packed | 15 cm × 4.6 mm × - & -/0–0.34/- | UV 6 | anilines (4), alkylbenzenes (5), PAHs 7 (5) & thioureas (3) | [101] |
| NH2-MIL-101(Al) (Al3+ & H2N-H2bdc 8) DMF 5 (40)/130/72 | p(GMA-co-EDMA) 9/NH2-MIL-101(Al) (monolithic composite)/ monolithic | 15 cm × 100 µm i.d. 10 × 375 µm o.d. 11 & 16,000/-/- | UV 6 | PAHs 7 (4) & NSAIDs 12 (3) | [102] |
| UiO-66-NH2 (Zr4+ & H2N-H2bdc 8) DMF 5 (20)/120/24 | pGMA 13/ UiO-66-NH2 (composite)/packed | 112 cm × 25 µm i.d. 10 × 365 µm o.d. 11 & 121,477/-/- | UV 6 | flavonoids (2) | [103] |
| UiO-66 (Zr4+ & H2bdc 14) DMF 5 (40) & acetic acid (4)/120/24 | UiO-66@SiO2 (core-shell composite)/packed | 15 cm × 4.6 mm × 5–6 µm & -/-/0.2–5.6 | UV 6 | xylene isomers (3) | [104] |
| MIL-101(Fe)-NH2 (Fe3+ & H2N-H2bdc 8) DMF 5 (13)/120/72 | MIL-101(Fe)-NH2@SiO2 (core-shell composite)/packed | 25 cm × 3 mm × 3–5 µm & 37,570/-/- | UV 6 | C8 compounds (5) | [105] |
| γ-CD-MOF (K+ & γ-CD 15) water (150) & methanol (15)/50/12 | γ-CD-MOF (composite)/packed | 10 cm × 4.6 mm × 2–5 µm & 75,000/ 2.59–13.96/- | DAD 16 | drugs (3) | [106] |
| ZIF-8 (Zn2+ & 2-MIm 17) DMF 5 (5.6) & methanol (14.4)/RT 18/24 | ZIF-8@SiO2 (core-shell composite)/packed | 5 cm × 4.6 mm × 2.2 µm & 216,202/-/- | UV 6 | xylene isomers (3) | [107] |
| [Nd3(D-cam)8(H2O)4Cl]n (Nd3+ & D-H2cam 19) methanol (60) & ACN (30) & water (30)/140/72 | [Nd3(D-cam)8(H2O)4Cl]n (composite)/packed | 25 cm × 2 mm × - & 9086/-/ 0.80–3.40 | UV 6 | racemates (27) | [108] |
| GC | |||||
| [Co-L-GG(H2O)] (Co2+ & L-GG 20) water (12) & methanol (12)/80/2 | [Co-L-GG(H2O)] (chiral)/coated | 10 m × 0.25 mm × 2 µm & 2790/0.54–4.29/1.04–1.34 | FID 21 | racemates (30) | [109] |
| [Zn2(bdc)(L-lac)(DMF)]·DMF (Zn2+ & L-lactic acid & H2bdc 14) DMF 5 (10)/120/48 | [Zn2(bdc)(L-lac)] (chiral)/coated | 10 m × 0.25 mm × 1–2 µm & -/-/- | FID 21 | biochemical compounds (12) | [110] |
| [Mn3(HCOO)2(D-cam)2(DMF)2]n (Mn2+ & D-H2cam 19)DMF 5 (5.5) & ethanol (3)/100/48 | [Mn3(HCOO)2(D-cam)2(DMF)2]n (chiral)/coated | 10 m × 0.25 mm × 1–2 µm & -/0.73–4.12/ 1.31–3.13 | FID 21 | biochemical compounds (9) | [111] |
| CEC | |||||
| UiO-66-NH2 (Zr4+ & H2N-H2bdc 8) DMF 5 (10) & formic acid (1)/120/24 | UiO-66-NH2 (functionalized)/ coated | 25 cm × 50 µm i.d. 10 × 365 µm o.d. 11 & -/-/- | DAD 16 | benzene derivatives (3) | [112] |
| Bio-MOF-1 (Zn2+ & H2bdpc 4 & adenine) DMF 5 (13.5) & water (1)/130/24 | bio-MOF-1 (functionalized)/ coated | 26.5 cm × 50 µm i.d. 10 × 360 µm o.d. 11 & 193000 | DAD 16 | chlorobenzenes (3) and alkylbenzenes (3) | [113] |
| [Mn(cam)(bpy) (Mn2+ & bpy & D-H2cam 19) DMF 5 (6) & ethanol (12)/100/72 | [Mn(cam)(bpy)] (functionalized)/ coated | 21 cm × 75 μm i.d. 10 × - & 118185 | DAD 16 | sulfonamides (10) | [114] |
| JLU-Liu23 (Cu+ & TEDA 23 &1,3-bis(2-benzimidazol)benzene) DMF 5 (0.75)/105/12 | JLU-Liu23 (functionalized)/ coated | 40 cm × 75 µm i.d. 10 × - & 194061 | DAD 16 | chiral neurotransmitters (4) | [115] |
| 1 highest theoretical plate number reported for the column 2 retention factor 3 selectivity factor 4 4,4’-biphenyldicarboxylic acid 5 N,N-dimethylformamide 6 ultraviolet detector 7 polycyclic aromatic hydrocarbons 8 2-aminoterephthalic acid (2-aminobenzene-1,4-dicarboxylic acid) 9 poly(glycidil methacrylate-co-ethylene dimethacrylate) 10 inner diameter 11 outer diameter | 12 non-steroidal anti-inflammatory drugs 13 poly(glycidil methacrylate) 14 terephthalic acid (benzene-1,4-dicarboxylic acid) 15 γ-cyclodextrin 16 diode array detector 17 2-methylimidazole 18 room temperature 19 D-(+)-camphoric acid 20 dipeptide H-Gly-L-Glu 21 flame ionization detector 22 4,4’-bipyridine 23 triethylenediamine -: non-reported | ||||
| MOF (metallic ion & ligand) Synthetic solvent (mL)/T (°C)/time (h) | MOF-based material/amount/comment | Method/ comments | Sample matrix (amount) | LOD 1/ RSD 2 (%) | Analytes (number) | Ref. | |
|---|---|---|---|---|---|---|---|
| Electroanalytical | |||||||
| ZIF-67 (Co2+ & 2-MIm 3) methanol (100)/RT 4/24 | neat ZIF-67/ 43 mg/CPE 5 electrode | amperometry/−50 mV applied | waters (-) | 1.45 µM/ <3.26 | hydrazine (1) | [121] | |
| PCN-224 (Zr4+ & TCPP 6) DMF 7 (50)/120/24 | PCN with rGO 8/ 5 mg·mL−1 suspension/ n-type semiconductor | EIS 9/ −100 mV applied | water, swine manure & lixivium (-) | 5.47 ng·L−1/ <3.8 | p-ASA (1) | [122] | |
| Cu-MOF (Cu2+ & H3btc 10) methanol (12) & water (12)/RT 4/2 | Cu-MOF with AuNPs 11/60 µL 10 µM solution/ aptasensor | DPV 12/−300–500 mV applied | aCSF 13 (-) | 0.45 nM/ 1.8 | β-amyloid oligomers | [123] | |
| Ni-BTC (Ni2+ & H3btc 10) ethanol (100) & water (100)/RT 4/0.5 | neat Ni-BTC/100 mg/CPE 5 electrode | CV 14/ 400–900 mV applied | soft drinks (-) | 0.08 nM/ <5 | Ponceau 4R (1) | [117] | |
| H2N-MIL-53(Al) (Al3+ & H2N-H2bdc 15) water (150)/reflux/72 | H2N-MIL-53(Al) in polymeric matrix/40 wt % MOF/ | EIS 9/1000 mV applied | - | -/- | methanol (1) & water (1), (in gas phase) | [124] | |
| Ce-MOF (Ce3+ & H3btc 10) ethanol (25) & water (25)/90/2 | Ce-MOF@MCA 16/ 1 mg/ aptasensor | EIS 9/- | milk, urine & water (-) | 17.4 fg·mL−1/ <2.65 | OTC 17 (1) | [125] | |
| Cu-MOF (Cu2+ & H3btc 10) ethanol (7.1) & water (7.1)/150/24 | Cu-MOF-GN 18/2 mg/GCE 19 electrode | CV 14 & DPV 12/ −0.4–0.8 V & −0.2–0.6 V | water (-) | 0.33–0.59 µM/ <2.8 | HQ 20 (1) & CT 21 (1) | [126] | |
| Spectroscopic | |||||||
| Mg-MOF 1 (Mg2+ & H2ATDC 22) DMF (4) & methanol (4) & water (2)/90/96 | neat Mg-MOF 1/-/pH and thermo stability | fluorescence/ Ksv 23 = 0.58 × 104 | - | 0.01–0.12 μM/- | Cr3+ (1) & NAEs 24 (8) | [118] | |
| Pr-MOF-NFs (Pr3+ & AIP 25 & Phen 26) DMF (2) & ethanol (18) & water (10)/reflux/48 | neat Pr-MOF-NFs 27/1 × 10−4 M/in distilled water | fluorescence/- | serum (-) | 0.276 ng·mL−1/ <1.42 | prolactin (1) | [42] | |
| Mg-APDA (Mg2+ & H2APDA 28) DMA 29 (2) & ethanol (0.1) & water (0.1)/140/48 | neat Mg-APDA/2.5 mg/in DMF 7 | fluorescence/Ksv 23 = 2.06 × 104 | - | 126–152 μg·L−1/- | Fe3+ (1), pesticides (5) & antibiotics (9) | [119] | |
| Zr6O4(OH)4(2,7-CDC)6]·19H2O·2DMF (Zr4+ & 2,7-H2CDC 30) & acetic acid (0.27)/80/24 | neat Zr-based MOF/2 mg/photostable and reusable | fluorescence/Ksv 23 = 5.5 × 104 | - | 0.02–0.91 μM | Fe3+ (1), CN- (1) & PNP 31 (1) | [127] | |
| Zn-MOF-1 (Zn2+ & H2bdc 32 & L 33) water (3) & ethanol (3)/170/72 | neat Zn-MOF-1/-/in methanol or water | fluorescence/Ksv 23 = 2.36 × 104 | - | 1.90–3.84 μM/- | Fe3+ (1), 2,6-Dich-4-NA 34 (1) & Cr6+ ions (2) | [128] | |
| Tb-MOF(Tb3+ & AIP 25) ethanol (40) & water (40)/RT/1 | Tb-MOF-PMMA 35/50 mg/MOF loaded in the polymeric membrane | fluorescence/ Ksv 23 = 4.0 × 104 | serum & water (-) | 0.30–0.35 μM/<4.7 | NFAs 36 (2) | [120] | |
| 1 limit of detection 2 relative standard deviation 3 2-methylimidazole 4 room temperature 5 carbon paste electrode 6 meso-tetra(4-carboxyphenyl)porphyrin 7N,N-dimethylformamide 8 reduced graphene oxide 9 electrochemical impedance spectroscopy 10 trimesic acid (benzene-1,3,5-tricarboxylic acid) 11 gold nanoparticles 12 differential pulse voltammetry 13 artificial cerebrospinal fluid 14 cyclic voltammetry 15 2-aminoterephthalic acid (2-aminobenzene-1,4-dicarboxylic acid) 16 melamine and cyanutic acidmonomers 17 oxytetracycline 18 graphene | 19 glassy carbon electrode 20 hydroquinone 21 catechol 22 2’-amino-1,1’:4’,1’’-terphenyl-4,4’’-dicarboxylic acid 23 highest quenching constant in M−1 24 nitro aromatic explosives 25 5-aminoisophthalate 26 phenanthroline 27 nanofibers 28 4,4’-(pyridine-3,5-diyl)dibenzoic acid 29 N,N-dimethylacetamide 30 9H-carbazole-2,7-dicarboxylic acid 31 p-nitrophenol 32 terephthalic acid (benzene-1,4-dicarboxylic acid) 33 4-(tetrazol-5-yl)phenyl-4,2’:6’,4’’-terpyridine 34 2,6-dichloro-4-nitroaniline 35 poly(methyl methacrylate) 36 nitrofuran antibiotics | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocío-Bautista, P.; Taima-Mancera, I.; Pasán, J.; Pino, V. Metal-Organic Frameworks in Green Analytical Chemistry. Separations 2019, 6, 33. https://doi.org/10.3390/separations6030033
Rocío-Bautista P, Taima-Mancera I, Pasán J, Pino V. Metal-Organic Frameworks in Green Analytical Chemistry. Separations. 2019; 6(3):33. https://doi.org/10.3390/separations6030033
Chicago/Turabian StyleRocío-Bautista, Priscilla, Iván Taima-Mancera, Jorge Pasán, and Verónica Pino. 2019. "Metal-Organic Frameworks in Green Analytical Chemistry" Separations 6, no. 3: 33. https://doi.org/10.3390/separations6030033
APA StyleRocío-Bautista, P., Taima-Mancera, I., Pasán, J., & Pino, V. (2019). Metal-Organic Frameworks in Green Analytical Chemistry. Separations, 6(3), 33. https://doi.org/10.3390/separations6030033