Determination of Avermectins Residues in Soybean, Bean, and Maize Using a QuEChERS-Based Method and Ultra-High-Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards, Chemicals, and Materials
2.2. UHPLC-MS/MS Parameters
2.3. Optimized Sample Preparation Procedure
2.4. Evaluation of the Different QuEChERS Procedures
2.5. Evaluation of Different Clean-Up Conditions
2.6. Validation Procedure
2.7. Application in Real Samples
3. Results
3.1. UHPLC-MS/MS Analysis
3.2. Sample Preparation Evaluations
3.3. Clean-Up Procedure Evaluations
3.4. Validation Results of the Proposed Method
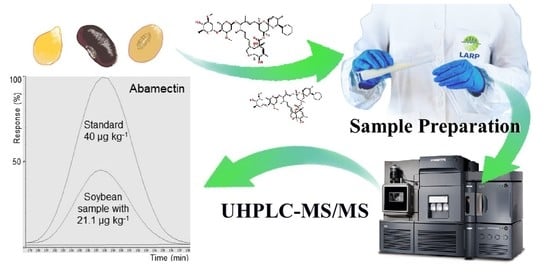
3.5. Application of the Method in Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Romanato, F.N.; Hamawaki, O.T.; de Sousa, L.B.; Nogueira, A.P.O.; de Neto, D.P.C.; Borges, C.C.R.; Hamawaki, C.D.L.; Hamawaki, R.L. Parametric and non-parametric analysis for determining the adaptability and stability of soybean genotypes in three sowing periods. Biosci. J. 2016, 32, 574–580. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Nan, X.; Yu, H.-T.; Cheng, P.-L.; Zhang, Y.; Liu, Y.-Q.; Zhang, S.-Y.; Hu, G.-F.; Liu, H.; Chen, A.-L. Synthesis, biological activities and structure activity relationships for new avermectin analogues. Eur. J. Med. Chem. 2016, 121, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.S.; Darwesh, D.M. Avermectins: The promising solution to control plant parasitic nematodes. J. Plant Sci. Phytopath. 2019, 3, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Brazil. Brazilian Ministry of Agriculture, Livestock and Supply. Normative Instruction nr. 13. Available online: https://www.infoconsult.com.br/legislacao/instrucao_normativa_mapa/2013/in_mapa_13_2013.htm (accessed on 14 October 2021).
- Bilal, M.; Freed, S.; Ashraf, M.Z.; Zaka, S.M.; Khan, M.B. Activity of acetylcholinesterase and acid and alkaline phosphatases in different insecticide-treated Helicoverpa armigera (Hübner). Environ. Sci. Pollut. Res. Int. 2018, 25, 22903–22910. [Google Scholar] [CrossRef]
- Bird, L.J.; Drynan, L.J.; Walker, P.W. The use of F2 screening for detection of resistance to emamectin benzoate, chlorantraniliprole, and indoxacarb in australian populations of Helicoverpa armígera (Lepidoptera: Noctuidae). J. Econ. Entomol. 2017, 110, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Orso, D.; Floriano, L.; Ribeiro, L.C.; Bandeira, N.M.G.; Prestes, O.D.; Zanella, R. Simultaneous determination of multiclass pesticides and antibiotics in honey samples based on ultra-high performance liquid chromatography-tandem mass spectrometry. Food Anal. Methods 2016, 9, 1638–1653. [Google Scholar] [CrossRef]
- May, M.M.; Ferronato, G.; Bandeira, N.M.G.; Prestes, O.D.; Zanella, R.; Adaime, M.A. Determination of pesticide residues in soy-based beverages using a QuEChERS method with clean-up optimized by central composite design and ultra-high-performance liquid chromatography-tandem mass spectrometry. Food Anal. Methods 2017, 10, 369–378. [Google Scholar] [CrossRef]
- Bandeira, N.M.G.; Ribeiro, L.C.; Rizzetti, T.M.; Martins, M.L.; Adaime, M.B.; Zanella, R.; Prestes, O.D. Evaluation of QuEChERS sample preparation for determination of avermectins residues in ovine muscle by HPLC-FD and UHPLC-MS/MS. J. Braz. Chem. Soc. 2017, 28, 878–886. [Google Scholar] [CrossRef]
- Musarurwa, H.; Chimuka, L.; Pakade, V.E.; Tavengwa, N.T. Recent developments and applications of QuEChERS based techniques on food samples during pesticide analysis. J. Food Compos. Anal. 2019, 84, 103314. [Google Scholar] [CrossRef]
- González-Curbelo, M.Á.; Herrera-Herrera, A.V.; Ravelo-Pérez, L.M.; Hernández-Borges, J. Sample-preparation methods for pesticide-residue analysis in cereals and derivatives. Trends Anal. Chem. 2012, 38, 32–51. [Google Scholar] [CrossRef]
- Huang, J.-X.; Lu, D.-H.; Wan, K.; Wang, F.-H. Low temperature purification method for the determination of abamectin and ivermectin in edible oils by liquid chromatography–tandem mass spectrometry. Chin. Chem. Lett. 2014, 25, 635–639. [Google Scholar] [CrossRef]
- Macedo, F.; Marsico, E.T.; Conte-Júnior, C.A.; de Resende, M.F.; Brasil, T.F.; Netto, A.D.P. Development and validation of a method for the determination of low-ppb levels of macrocyclic lactones in butter, using HPLC-fluorescence. Food Chem. 2015, 179, 239–245. [Google Scholar] [CrossRef]
- López-Blanco, R.; Nortes-Méndez, R.; Robles-Molina, J.; Moreno-Gonzáles, D.; Gilbert-López, B.; García-Reyes, J.F.; Molina-Díaz, A. Evaluation of different cleanup sorbents for multiresidue pesticide analysis in fatty vegetable matrices by liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1456, 89–104. [Google Scholar] [CrossRef]
- Du, P.; Liu, X.; Gu, X.; Dong, F.; Xu, J.; Kong, Z.; Wu, Y.; Zhu, Y.; Li, Y.; Zheng, Y. Rapid residue analysis of pyriproxyfen, avermectins and diflubenzuron in mushrooms by ultra-performance liquid chromatography coupled with tandem mass spectrometry. Anal. Methods 2013, 5, 6741–6747. [Google Scholar] [CrossRef]
- Liu, Z.; Qi, P.; Wang, X.; Wang, Z.; Xu, X.; Chen, W.; Wu, L.; Zhang, H.; Wang, Q.; Wang, X. Multi-pesticides residue analysis of grains using modified magnetic nanoparticle adsorbent for facile and efficient cleanup. Food Chem. 2017, 230, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheung, W.; Chow, W. Ultra-high performance liquid chromatography/ electrospray ionization-tandem mass spectrometry determination of 151 pesticides in soybeans and pulses. J. AOAC Int. 2013, 96, 1114–1133. [Google Scholar] [CrossRef] [PubMed]
- Agilent Technologies. Enhanced Matrix Removal-Lipid Brochure; 5991-6052EN; Agilent Technologies: Santa Clara, CA, USA, 2016. [Google Scholar]
- Anastassiades, M.; Lehotay, S.; Štajnbaher, D.; Schenck, F. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anastassiades, M.; Scherbaum, E.; Tasdelen, B.; Stajnbaher, D. Recent developments in QuEChERS methodology for pesticide multiresidue analysis. In Pesticide Chemistry: Crop Protection, Public Health, Environmental Safety, 1st ed.; Ohkawa, H.M., Hisashi, L., Philip, W., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 439–458. [Google Scholar] [CrossRef]
- Lehotay, S.J.; Mastovská, K.; Lightfield, A.R. Use of buffering and other means to improve results of problematic pesticides in a fast and easy method for residue analysis of fruits and vegetables. J. AOAC Int. 2005, 88, 615–629. [Google Scholar] [CrossRef] [Green Version]
- European Commission. SANTE/12682/2019. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed. European Commission Directorate-General for Health and Food Safety. (rev.0). 2019. Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/AqcGuidance_SANTE_2019_12682.pdf (accessed on 14 October 2021).
- Kemmerich, M.; Rizetti, T.M.; Martins, M.L.; Prestes, O.D.; Adaime, M.B.; Zanella, R. Optimization by central composite design of a modified QuEChERS method for extraction of pesticide multiresidue ins sweet pepper and analysis by ultra-high-performance liquid chromatography–tandem mass spectrometry. Food Anal. Methods 2015, 8, 728–739. [Google Scholar] [CrossRef]
- Moscou, I.C.; Dasenaki, M.E.; Thomaidis, N.S. Ionization study and simultaneous determination of avermectins and milbemycines in fish tissue by LC-ESI-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019, 1104, 134–140. [Google Scholar] [CrossRef]
- Marchis, D.; Ferro, G.L.; Brizio, P.; Squadrone, S.; Abete, M.C. Detection of pesticides in crops: A modified QuEChERS approach. Food Control 2012, 25, 270–273. [Google Scholar] [CrossRef]
- Herrmann, S.S.; Poulsen, M.E. Clean-up of cereal extracts for gas chromatography-tandem quadrupole mass spectrometry pesticide residues analysis using primary secondary amine and C18. J. Chromatogr. A 2015, 1423, 47–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francesquett, J.Z.; Rizzetti, T.M.; Cadaval, T.R.S.; Prestes, O.D.; Adaime, M.B.; Zanella, R. Simultaneous determination of the quaternary ammonium pesticides paraquat, diquat, chlormequat, and mepiquat in barley and wheat using a modified quick polar pesticides method, diluted standard addition calibration and hydrophilic interaction liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2019, 1592, 101–111. [Google Scholar] [CrossRef]
- Menezes Filho, A.; dos Santos, F.N.; Pereira, P.A.P. Development, validation and application of a methodology based on solid-phase micro extraction followed by gas chromatography coupled to mass spectrometry (SPME/GC-MS) for the determination of pesticide residues in mangoes. Talanta 2010, 81, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Seth, S.; Agrawal, Y.C.; Ghosh, P.K.; Jayas, D.S.; Singh, B.P.N. Oil extraction rates of soya bean using isopropyl alcohol as solvent. Biosys. Eng. 2007, 97, 209–217. [Google Scholar] [CrossRef]


| Compounds | Log Kow | tR (min) | Precursor Ion | Cone (eV) | SRM Transitions, m/z (Collision Energy, eV) | Ion Ratio | ||
|---|---|---|---|---|---|---|---|---|
| Quantification | Identification | |||||||
| Abamectin |  | 4.0 | 2.83 | [M + NH4]+ | 20 | 890.6 > 567.4 (11) | 890.6 > 305.2 (25) | 0.91 |
| Doramectin |  | 4.0 | 3.02 | [M + NH4]+ | 15 | 916.6 > 331.2 (23) | 916.6 > 219.1 (25) | 0.81 |
| Emamectin benzoate |  | 3.0 | 2.23 | [M + H]+ | 40 | 886.6 > 158.0 (37) | 886.6 > 126.0 (38) | 0.62 |
| Eprinomectin |  | 5.4 | 2.72 | [M + H]+ | 15 | 914.5 > 186.0 (35) | 914.5 > 144.0 (20) | 0.67 |
| Ivermectin |  | 3.2 | 3.23 | [M + NH4]+ | 15 | 892.6 > 569.4 (14) | 892.6 > 551.4 (25) | 0.42 |
| Compounds | MRL (µg kg−1) | Recovery (RSD) (%) for 5 Spike Levels (µg kg−1) (n = 5) | ME (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 8 | 20 | 40 | 80 | |||||||||
| BR | CA | EU | USA | Repeat. | Interm. Prec. | Repeat. | Interm. prec. | Repeat. | Repeat. | Interm. Prec. | Repeat. | ||
| Soybean | |||||||||||||
| Abamectin | 10 | 10 | 10 | 77 (11) | 98 (17) | 79 (5) | 101 (13) | 114 (14) | 102 (12) | 94 (13) | 99 (10) | −7 | |
| Doramectin | 109 (13) | 100 (15) | 101 (9) | 100 (8) | 99 (10) | 99 (14) | 102 (6) | 100 (5) | −16 | ||||
| Emamectin | 10 | 10 | 88 (7) | 106 (9) | 97 (5) | 96 (6) | 103 (7) | 101 (9) | 105 (5) | 100 (9) | −4 | ||
| Eprinomectin | 102 (16) | 87 (16) | 98 (17) | 117 (8) | 113 (19) | 91 (17) | 105 (3) | 101 (13) | −6 | ||||
| Ivermectin | 105 (4) | 98 (14) | 104 (3) | 89 (9) | 104 (15) | 94 (11) | 102 (6) | 101 (9) | −11 | ||||
| Bean | |||||||||||||
| Abamectin | 5 | 80 | 10 | 15 | 83 (5) | 98 (13) | 89 (6) | 97 (4) | 105 (10) | 103 (10) | 96 (11) | 99 (9) | 1 |
| Doramectin | 71 (2) | 108 (7) | 107 (3) | 95 (10) | 91 (5) | 110 (6) | 96 (4) | 98 (7) | −11 | ||||
| Emamectin | 10 | 10 | 10 | 105 (9) | 108 (6) | 94 (12) | 95 (10) | 105 (14) | 104 (5) | 103 (6) | 99 (8) | 1 | |
| Eprinomectin | 112 (6) | 89 (14) | 103 (16) | 106 (15) | 90 (11) | 96 (10) | 95 (6) | 101 (11) | 0.2 | ||||
| Ivermectin | 101 (5) | 93 (12) | 98 (3) | 104 (19) | 98 (7) | 102 (4) | 95 (9) | 100 (11) | −4 | ||||
| Maize | |||||||||||||
| Abamectin | 5 | 10 | 71 (6) | 74 (5) | 106 (6) | 109 (10) | 92 (15) | 107 (8) | 97 (6) | 99 (9) | −6 | ||
| Doramectin | 118 (3) | 105 (11) | 113 (7) | 97 (5) | 88 (7) | 100 (6) | 96 (6) | 101 (11) | 22 | ||||
| Emamectin | 10 | 10 | 89 (16) | 103 (12) | 105 (1) | 98 (6) | 109 (3) | 104 (4) | 103 (3) | 99 (3) | −10 | ||
| Eprinomectin | 96 (5) | 94 (17) | 117 (9) | 99 (11) | 89 (12) | 103 (13) | 98 (8) | 100 (19) | −16 | ||||
| Ivermectin | 102 (5) | 115 (4) | 102 (3) | 92 (9) | 87 (1) | 109 (3) | 101 (2) | 99 (8) | 14 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uczay, F.; Bandeira, N.M.G.; Floriano, L.; Prestes, O.D.; Adaime, M.B.; Zanella, R. Determination of Avermectins Residues in Soybean, Bean, and Maize Using a QuEChERS-Based Method and Ultra-High-Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. Separations 2021, 8, 214. https://doi.org/10.3390/separations8110214
Uczay F, Bandeira NMG, Floriano L, Prestes OD, Adaime MB, Zanella R. Determination of Avermectins Residues in Soybean, Bean, and Maize Using a QuEChERS-Based Method and Ultra-High-Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. Separations. 2021; 8(11):214. https://doi.org/10.3390/separations8110214
Chicago/Turabian StyleUczay, Fernanda, Nelson M. G. Bandeira, Luana Floriano, Osmar D. Prestes, Martha B. Adaime, and Renato Zanella. 2021. "Determination of Avermectins Residues in Soybean, Bean, and Maize Using a QuEChERS-Based Method and Ultra-High-Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry" Separations 8, no. 11: 214. https://doi.org/10.3390/separations8110214
APA StyleUczay, F., Bandeira, N. M. G., Floriano, L., Prestes, O. D., Adaime, M. B., & Zanella, R. (2021). Determination of Avermectins Residues in Soybean, Bean, and Maize Using a QuEChERS-Based Method and Ultra-High-Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. Separations, 8(11), 214. https://doi.org/10.3390/separations8110214