Application of Humic and Fulvic Acids as an Alternative Method of Cleaning Water from Plant Protection Product Residues
Abstract
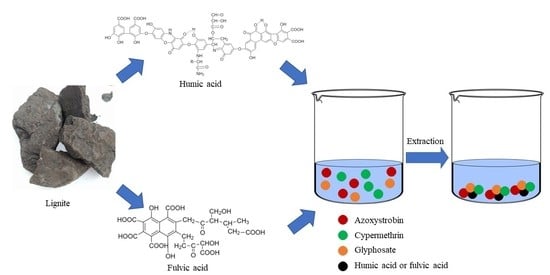
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation
2.3. Proximate Composition of the Examined Lignites
2.4. HA and FA Isolation
2.5. Removal of Organic Compounds
2.6. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition of the Used Lignites
3.2. Characterization of the Sorbents
3.3. Sorption Study
3.3.1. Glyphosate Sorption
3.3.2. Cypermethrin Sorption
3.3.3. Azoxystrobin Sorption
3.3.4. Leaching Study
3.3.5. Sorption from Lake Water
3.4. Tentative Sorption Mechanisms
3.4.1. Glyphosate Sorption Tentative Mechanism
3.4.2. Cypermethrin Sorption Tentative Mechanism
3.4.3. Azoxystrobin Sorption Tentative Mechanisms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mojid, M.A.; Mainuddin, M.; Murad, K.F.I.; Kirby, J. Mac Water usage trends under intensive groundwater-irrigated agricultural development in a changing climate—Evidence from Bangladesh. Agric. Water Manag. 2021, 251, 106873. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Adeniyi, A.G.; Adelodun, A.A. Recent advances on the adsorption of herbicides and pesticides from polluted waters: Performance evaluation via physical attributes. J. Ind. Eng. Chem. 2021, 93, 117–137. [Google Scholar] [CrossRef]
- Gupta, P.K. Herbicides and Fungicides. In Reproductive and Developmental Toxicology; Gupta, R.C., Ed.; Academic Press: Burlington, MA, USA, 2011; pp. 503–521. [Google Scholar] [CrossRef]
- Gandhi, K.; Khan, S.; Patrikar, M.; Markad, A.; Kumar, N.; Choudhari, A.; Sagar, P.; Indurkar, S. Exposure risk and environmental impacts of glyphosate: Highlights on the toxicity of herbicide co-formulants. Environ. Chall. 2021, 4, 100149. [Google Scholar] [CrossRef]
- Ansari, M.; Hatami, B.; Khavidak, S.S. Toxicity, Biodegradability and Detection Methods of Glyphosate; the Most Used Herbicide: A Systematic Review. J. Environ. Health Sustain. Dev. 2019, 4, 731–743. [Google Scholar] [CrossRef]
- Annett, R.; Habibi, H.R.; Hontela, A. Impact of glyphosate and glyphosate-based herbicides on the freshwater environment. J. Appl. Toxicol. 2014, 34, 458–479. [Google Scholar] [CrossRef] [PubMed]
- Ponnuchamy, M.; Kapoor, A.; Senthil Kumar, P.; Vo, D.V.N.; Balakrishnan, A.; Mariam Jacob, M.; Sivaraman, P. Sustainable adsorbents for the removal of pesticides from water: A review. Environ. Chem. Lett. 2021, 19, 2425–2463. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Vasilas, A.; Chatzimitakos, T.; Sygellou, L.; Stalikas, C. Performance study of a magnetic iron–copper bimetallic material for the removal of an environmental “cocktail” of diverse hazardous organic micropollutants from aqueous samples. Nanotechnol. Environ. Eng. 2021, 6, 66. [Google Scholar] [CrossRef]
- Fallah, Z.; Zare, E.N.; Ghomi, M.; Ahmadijokani, F.; Amini, M.; Tajbakhsh, M.; Arjmand, M.; Sharma, G.; Ali, H.; Ahmad, A.; et al. Toxicity and remediation of pharmaceuticals and pesticides using metal oxides and carbon nanomaterials. Chemosphere 2021, 275, 130055. [Google Scholar] [CrossRef]
- Rawtani, D.; Khatri, N.; Tyagi, S.; Pandey, G. Nanotechnology-based recent approaches for sensing and remediation of pesticides. J. Environ. Manag. 2018, 206, 749–762. [Google Scholar] [CrossRef]
- Yarima, A.; Ali, R.; Abdullahi, A.A.; Idris, Z. Nanotechnology: Review on Emerging Techniques in Remediating Water and Soil Pollutions. J. Appl. Sci. Environ. Manag. 2020, 24, 933–941. [Google Scholar] [CrossRef]
- Madannejad, R.; Shoaie, N.; Jahanpeyma, F.; Darvishi, M.H.; Azimzadeh, M.; Javadi, H. Toxicity of carbon-based nanomaterials: Reviewing recent reports in medical and biological systems. Chem. Biol. Interact. 2019, 307, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Arroyave, J.M.; Waiman, C.C.; Zanini, G.P.; Avena, M.J. Effect of humic acid on the adsorption/desorption behavior of glyphosate on goethite. Isotherms and kinetics. Chemosphere 2016, 145, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Bouras, O.; Bollinger, J.C.; Baudu, M. Effect of humic acids on pentachlorophenol sorption to cetyltrimethylammonium-modified, Fe- and Al-pillared montmorillonites. Appl. Clay Sci. 2010, 50, 58–63. [Google Scholar] [CrossRef]
- Bollag, J.M.; Myers, C.J.; Minard, R.D. Biological and chemical interactions of pesticides with soil organic matter. Sci. Total Environ. 1992, 123–124, 205–217. [Google Scholar] [CrossRef]
- Khan, S.; Khan, N.N. The mobility of some organophosphorus pesticides in soils as affected by some soil parameters. Soil Sci. 1986, 142, 214–222. [Google Scholar] [CrossRef]
- De Paolis, F.; Kukkonen, J. Binding of organic pollutants to humic and fulvic acids: Influence of pH and the structure of humic material. Chemosphere 1997, 34, 1693–1704. [Google Scholar] [CrossRef]
- Guo, X.; Tu, B.; Ge, J.; Yang, C.; Song, X.; Dang, Z. Sorption of tylosin and sulfamethazine on solid humic acid. J. Environ. Sci. 2016, 43, 208–215. [Google Scholar] [CrossRef]
- Vrantsi, E.; Lakka, A.; Bozinou, E.; Athanasiadis, V.; Papadaki, E.S.; Dourtoglou, V.G.; Lalas, S.I. Humic and Fulvic Acids as Specific Sorbents of Herbicides in Water. Clean—Soil Air Water 2021, 49, 2000467. [Google Scholar] [CrossRef]
- Zhou, F.; Cheng, J.; Liu, J.; Zhou, J.; Cen, K. Improving physicochemical properties of upgraded Indonesian lignite through microwave irradiation with char adsorbent. Fuel 2018, 218, 275–281. [Google Scholar] [CrossRef]
- Badour, C.; Gilbert, A.; Xu, C.; Li, H.; Shao, Y.; Tourigny, G.; Preto, F. Combustion and air emissions from co-firing a wood biomass, a Canadian peat and a Canadian lignite coal in a bubbling fluidised bed combustor. Can. J. Chem. Eng. 2012, 90, 1170–1177. [Google Scholar] [CrossRef]
- Wang, S.; Mulligan, C.N. Effect of natural organic matter on arsenic release from soils and sediments into groundwater. Environ. Geochem. Health 2006, 28, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Yang, H.; Tong, L.; Zhong, S.; Liu, Y. Spectral study of humic substance extract from pressurized oxidizing slag of Carlin-typed gold deposit. In Proceedings of the Journal of Physics: Conference Series, Sochi, Russia, 16–19 October 2019; Volume 1347. [Google Scholar]
- Kar, S.; Maity, J.P.; Jean, J.S.; Liu, C.C.; Nath, B.; Lee, Y.C.; Bundschuh, J.; Chen, C.Y.; Li, Z. Role of organic matter and humic substances in the binding and mobility of arsenic in a Gangetic aquifer. J. Environ. Sci. Health—Part A Toxic/Hazard. Subst. Environ. Eng. 2011, 46, 1231–1238. [Google Scholar] [CrossRef]
- Wu, M.; Song, M.; Liu, M.; Jiang, C.; Li, Z. Fungicidal activities of soil humic/fulvic acids as related to their chemical structures in greenhouse vegetable fields with cultivation chronosequence. Sci. Rep. 2016, 6, 32858. [Google Scholar] [CrossRef]
- Marin-Morales, M.A.; de Campos Ventura-Camargo, B.; Hoshina, M.M. Toxicity of Herbicides: Impact on Aquatic and Soil Biota and Human Health. In Herbicides—Current Research and Case Studies in Use; Price, A.J., Kelton, J.A., Eds.; IntechOpen: London, UK, 2013; pp. 399–443. [Google Scholar] [CrossRef] [Green Version]
- Mesnage, R.; Antoniou, M.N. Facts and Fallacies in the Debate on Glyphosate Toxicity. Front. Public Health 2017, 5, 316. [Google Scholar] [CrossRef] [Green Version]
- Mayakaduwa, S.S.; Kumarathilaka, P.; Herath, I.; Ahmad, M.; Al-Wabel, M.; Ok, Y.S.; Usman, A.; Abduljabbar, A.; Vithanage, M. Equilibrium and kinetic mechanisms of woody biochar on aqueous glyphosate removal. Chemosphere 2016, 144, 2516–2521. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ouyang, Z.; Zhang, Z.; Yang, C.; Li, X.; Dang, Z.; Wu, P. Mechanism of glyphosate removal by biochar supported nano-zero-valent iron in aqueous solutions. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 547, 64–72. [Google Scholar] [CrossRef]
- Hu, Y.S.; Zhao, Y.Q.; Sorohan, B. Removal of glyphosate from aqueous environment by adsorption using water industrial residual. Desalination 2011, 271, 150–156. [Google Scholar] [CrossRef]
- Yadav, B. Cypermethrin Toxicity: A Review. J. Forensic Sci. Crim. Investig. 2018, 9, 555767. [Google Scholar] [CrossRef]
- Tallur, P.N.; Megadi, V.B.; Ninnekar, H.Z. Biodegradation of Cypermethrin by Micrococcus sp. strain CPN 1. Biodegradation 2008, 19, 77–82. [Google Scholar] [CrossRef]
- Al-Smadi, B.M.; Al Oran, E.H.; Abu Hajar, H.A. Adsorption-desorption of cypermethrin and chlorfenapyr on Jordanian soils. Arab. J. Geosci. 2019, 12, 465. [Google Scholar] [CrossRef]
- Domingues, V.F.; Priolo, G.; Alves, A.C.; Cabral, M.F.; Delerue-Matos, C. Adsorption behavior of α-cypermethrin on cork and activated carbon. J. Environ. Sci. Health—Part B Pestic. Food Contam. Agric. Wastes 2007, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Năstuneac, V.; Panainte-Lehăduş, M.; Moşneguţu, E.F.; Gavrilaş, S.; Cioca, G.; Munteanu, F.D. Removal of cypermethrin fromwater by using fucus spiralis marine alga. Int. J. Environ. Res. Public Health 2019, 16, 3663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, E.T.; Lopes, I.; Pardal, M.Â. Occurrence, fate and effects of azoxystrobin in aquatic ecosystems: A review. Environ. Int. 2013, 53, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Hai, N.; Liu, X.; Li, Y.; Kong, F.; Zhang, Y.; Fang, S. Effects of Microplastics on the Adsorption and Bioavailability of Three Strobilurin Fungicides. ACS Omega 2020, 5, 30679–30686. [Google Scholar] [CrossRef]
- Sil, A.; Narayanan, N.; Gupta, S. Removal of Pesticides from Water using Magnetite-Activated Charcoal. Pestic. Res. J. 2022, 34, 78–84. [Google Scholar] [CrossRef]
- Ćwieląg-Piasecka, I.; Medyńska-Juraszek, A.; Jerzykiewicz, M.; Dębicka, M.; Bekier, J.; Jamroz, E.; Kawałko, D. Humic acid and biochar as specific sorbents of pesticides. J. Soils Sediments 2018, 18, 2692–2702. [Google Scholar] [CrossRef] [Green Version]
- Chatzimitakos, T.G.; Karali, K.K.; Stalikas, C.D. Magnetic graphene oxide as a convenient nanosorbent to streamline matrix solid-phase dispersion towards the extraction of pesticides from vegetables and their determination by GC–MS. Microchem. J. 2019, 151, 104247. [Google Scholar] [CrossRef]
- Mazzei, P.; Piccolo, A. Quantitative evaluation of noncovalent interactions between glyphosate and dissolved humic substances by NMR spectroscopy. Environ. Sci. Technol. 2012, 46, 5939–5946. [Google Scholar] [CrossRef]
- Zhou, J.L.; Rowland, S.J.; Mantoura, R.F.C.; Harland, B.J. Influence of the nature of particulate organic matter on the sorption of cypermethrin: Implications on KOC correlations. Environ. Int. 1995, 21, 187–195. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makrigianni, E.A.; Papadaki, E.S.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Lalas, S.I. Application of Humic and Fulvic Acids as an Alternative Method of Cleaning Water from Plant Protection Product Residues. Separations 2022, 9, 313. https://doi.org/10.3390/separations9100313
Makrigianni EA, Papadaki ES, Chatzimitakos T, Athanasiadis V, Bozinou E, Lalas SI. Application of Humic and Fulvic Acids as an Alternative Method of Cleaning Water from Plant Protection Product Residues. Separations. 2022; 9(10):313. https://doi.org/10.3390/separations9100313
Chicago/Turabian StyleMakrigianni, Eirini A., Eirini S. Papadaki, Theodoros Chatzimitakos, Vassilis Athanasiadis, Eleni Bozinou, and Stavros I. Lalas. 2022. "Application of Humic and Fulvic Acids as an Alternative Method of Cleaning Water from Plant Protection Product Residues" Separations 9, no. 10: 313. https://doi.org/10.3390/separations9100313
APA StyleMakrigianni, E. A., Papadaki, E. S., Chatzimitakos, T., Athanasiadis, V., Bozinou, E., & Lalas, S. I. (2022). Application of Humic and Fulvic Acids as an Alternative Method of Cleaning Water from Plant Protection Product Residues. Separations, 9(10), 313. https://doi.org/10.3390/separations9100313