A Neglected Issue: Stationary Phase Retention Determination of Classic High-Speed Counter-Current Chromatography Solvent Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Apparatus
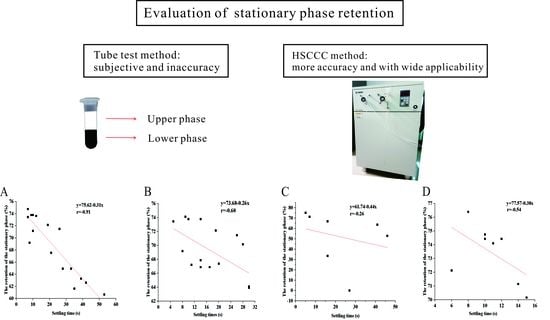
2.3. Settling Time Evaluation
2.4. Enrichment of Myricanol from the Bark of Myrica rubra (Lour.) Siebold & Zucc
2.5. The Relationship between Resolution and the Retention of the Stationary Phase
2.6. Measurement of the Retention of the Stationary Phase
3. Results
3.1. Separation of Myricanol from the Ethylacetate Extract of the Bark of M. rubra and the Relationship between Resolution and the Retention of the Stationary Phase
3.2. The Retention of the Stationary Phase of a Set of Classical Two-Phase Solvent Systems
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, F.; Cheng, L.; Cao, Y.; Wei, Q.; Tong, C.; Shi, S. Online extraction and enrichment coupling with high-speed counter-current chromatography for effective and target isolation of antitumor anthraquinones from seeds of Cassia obtusifolia. J. Sep. Sci. 2022, 45, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Hattori, Y.; Hino, T.; Oka, H. An approach to on-line electrospray mass spectrometric detection of poly-peptide antibiotics of enramycin for high-speed counter-current chromatographic separation. J. Pharm. Biomed. 2010, 51, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.; He, X.; Xu, P.; Zhang, B.; Ding, L. Rapid separation and purification of two C25 steroids with bicyclic [4.4.1] A/B rings from the marine fungus Aspergillus sp. LS116 by high-speed counter-current chromatography in stepwise elution mode. Nat. Prod. Res. 2021, 36, 3770–3774. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; He, X.; Wang, T.; Zhang, X.; Li, Z.; Xu, X.; Zhang, W.; Yan, X.; Li, S.; He, S. Efficient preparation of bafilo-mycin a1 from marine streptomyces lohii fermentation using three-phase extraction and high-speed counter-current chromatography. Mar. Drugs 2020, 18, 332. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Chen, F. Simultaneous separation and purification of five bioactive coumarins from the Chinese medicinal plant Cnidium monnieri by high-speed counter-current chromatography. J. Sep. Sci. 2005, 28, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A. 2005, 1065, 145–168. [Google Scholar] [CrossRef] [PubMed]
- Oka, F.; Oka, H.; Ito, Y. Systematic search for suitable two-phase solvent systems for high-speed counter-current chromatography. J. Chromatogr. A. 1991, 538, 99–108. [Google Scholar] [CrossRef]
- Foucault, A.P. Solvent systems in centrifugal partition chromatography. Chromatogr. Sci. Ser. 1995, 68, 71–98. [Google Scholar]
- Liu, Y.; Kuang, P.; Guo, S.; Sun, Q.; Xue, T.; Li, H. An overview of recent progress in solvent systems, additives and modifiers of counter current chromatography. New. J. Chem. 2018, 42, 6584–6600. [Google Scholar] [CrossRef]
- He, C.H.; Zhao, C.X. Retention of the stationary phase for high-speed countercurrent chromatography. AIChE J. 2007, 53, 1460–1471. [Google Scholar] [CrossRef]
- Friesen, J.B.; Pauli, G.F. GUESSmix-guided optimization of elution–extrusion counter-current separations. J. Chromatogr. A. 2009, 1216, 4225–4231. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Cheng, Q.; Zhang, P.; Tang, K.; Huang, Y.; Xu, W. Preparative enantioseparation of 2-(4-hydroxyphenyl) propionic acid by high speed counter-current chromatography with hydroxyethyl-β-cyclodextrin as chiral selector. Sep. Sci. Technol. 2018, 53, 2981–2989. [Google Scholar] [CrossRef]
- Berthod, A.; Schmitt, N. Water—Organic solvent systems in countercurrent chromatography: Liquid stationary phase retention and solvent polarity. Talanta 1993, 40, 1489–1498. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chinese Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Liu, Y.; Che, F.; Li, H.; Liu, J.; Wu, N.; Gu, Y.; Wei, Y. Isolation and purification of flavonoids from Euonymus alatus by high-speed countercurrent chromatography and neuroprotective effect of rhamnazin-3-O-rutinoside in vitro. J. Sep. Sci. 2021, 44, 4422–4430. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, L.; Yan, H.; Cui, L.; Hussain, H.; Xie, L.; Liu, J.; Jiang, Y.J.; Meng, Z.Q.; Cao, G.Y.; et al. An efficient high-speed counter-current chromatography method for the preparative separation of potential antioxidants from Paeonia lactiflora Pall. combination of in vitro evaluation and molecular docking. J. Sep. Sci. 2022, 45, 1856–1865. [Google Scholar] [CrossRef] [PubMed]


| No | nC6H14 | EtOAc | nBuOH | MeOH | H2O | VR (U/L) | ST (s) | MP | RR (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 0 | 0 | 5 | 5 | 1.06 | 5 ± 2 | Lower Phase | 73.44 ± 2.15 |
| 2 | 9 | 1 | 0 | 5 | 5 | 0.96 | 9 ± 3 | Lower Phase | 74.10 ± 3.28 |
| 3 | 8 | 2 | 0 | 5 | 5 | 0.89 | 14 ± 3 | Lower Phase | 73.77 ± 3.71 |
| 4 | 7 | 3 | 0 | 5 | 5 | 0.82 | 19 ± 3 | Lower Phase | 72.13 ± 2.81 |
| 5 | 6 | 4 | 0 | 5 | 5 | 0.77 | 10 ± 2 | Lower Phase | 73.77 ± 2.39 |
| 6 | 5 | 5 | 0 | 5 | 5 | 0.75 | 26 ± 3 | Lower Phase | 71.48 ± 2.91 |
| 7 | 4 | 5 | 0 | 4 | 5 | 0.80 | 28 ± 4 | Lower Phase | 70.16 ± 2.27 |
| 8 | 3 | 5 | 0 | 3 | 5 | 0.85 | 30 ± 3 | Lower Phase | 63.93 ± 2.70 |
| 9 | 2 | 5 | 0 | 2 | 5 | 0.94 | 30 ± 4 | Lower Phase | 63.91 ± 3.19 |
| 10 | 1 | 5 | 0 | 1 | 5 | 0.92 | 30 ± 3 | Lower Phase | 64.10 ± 1.81 |
| 11 | 0 | 5 | 0 | 0 | 5 | 0.89 | 8 ± 1 | Lower Phase | 69.18 ± 2.80 |
| 12 | 0 | 4 | 1 | 0 | 5 | 1.00 | 20 ± 3 | Lower Phase | 67.38 ± 2.29 |
| 13 | 0 | 3 | 2 | 0 | 5 | 1.11 | 14 ± 2 | Lower Phase | 67.87 ± 2.70 |
| 14 | 0 | 2 | 3 | 0 | 5 | 1.20 | 11 ± 2 | Lower Phase | 67.21 ± 1.90 |
| 15 | 0 | 1 | 4 | 0 | 5 | 1.30 | 14 ± 3 | Lower Phase | 66.89 ± 2.16 |
| 16 | 0 | 0 | 5 | 0 | 5 | 1.27 | 17 ± 2 | Lower Phase | 66.82 ± 2.82 |
| No | nC6H14 | EtOAc | MeOH | H2O | VR (U/L) | ST (s) | MP | RR (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 9 | 1 | 9 | 1 | 0.73 | 7 | Lower Phase | 74.75 ± 2.11 |
| 2 | 8 | 2 | 8 | 2 | 0.72 | 9 | Lower Phase | 73.77 ± 1.82 |
| 3 | 7 | 3 | 7 | 3 | 0.69 | 10 | Lower Phase | 71.15 ± 2.59 |
| 4 | 7 | 3 | 6 | 4 | 0.75 | 7 | Lower Phase | 73.44 ± 1.97 |
| 5 | 6 | 4 | 6 | 4 | 0.68 | 12 | Lower Phase | 73.61 ± 2.77 |
| 6 | 7 | 3 | 5 | 5 | 0.82 | 19 | Lower Phase | 72.13 ± 3.10 |
| 7 | 6 | 4 | 5 | 5 | 0.77 | 10 | Lower Phase | 73.77 ± 2.74 |
| 8 | 5 | 5 | 5 | 5 | 0.75 | 26 | Lower Phase | 71.48 ± 2.93 |
| 9 | 4 | 6 | 5 | 5 | 0.69 | 21 | Lower Phase | 67.54 ± 2.27 |
| 10 | 3 | 7 | 5 | 5 | 0.66 | 35 | Lower Phase | 61.64 ± 2.90 |
| 11 | 4 | 6 | 4 | 6 | 0.82 | 28 | Lower Phase | 64.92 ± 1.84 |
| 12 | 3 | 7 | 4 | 6 | 0.83 | 42 | Lower Phase | 62.62 ± 2.05 |
| 13 | 3 | 7 | 3 | 7 | 0.90 | 39 | Lower Phase | 63.28 ± 2.43 |
| 14 | 2 | 8 | 2 | 8 | 0.94 | 53 | Lower Phase | 60.66 ± 2.95 |
| 15 | 1 | 9 | 1 | 9 | 0.96 | 33 | Lower Phase | 64.92 ± 2.00 |
| 16 | 0 | 10 | 0 | 10 | 0.89 | 8 | Lower Phase | 69.18 ± 1.79 |
| No | CHCl3 | MeOH | H2O | VR (L/U) | ST (s) | MP | RR (%) |
|---|---|---|---|---|---|---|---|
| 1 | 10 | 0 | 10 | 0.98 | 6 | Lower Phase | 72.13 ± 1.66 |
| 2 | 10 | 1 | 9 | 1.00 | 8 | Lower Phase | 76.39 ± 2.00 |
| 3 | 10 | 2 | 8 | 1.04 | 12 | Lower Phase | 74.43 ± 2.38 |
| 4 | 10 | 3 | 7 | 1.06 | 14 | Lower Phase | 71.15 ± 1.97 |
| 5 | 10 | 4 | 6 | 1.09 | 10 | Lower Phase | 74.45 ± 2.70 |
| 6 | 10 | 5 | 5 | 1.15 | 11 | Lower Phase | 74.10 ± 2.50 |
| 7 | 10 | 6 | 4 | 1.27 | 10 | Lower Phase | 74.43 ± 2.82 |
| 8 | 10 | 7 | 3 | 1.86 | 15 | Lower Phase | 70.16 ± 1.92 |
| 9 | 10 | 8 | 2 | - | - | - | - |
| No | nC7H16 | nBuOH | ACN | H2O | VR(U/L) | ST(s) | MP | RR(%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 0 | 5 | 0 | 0.82 | 5 | Lower Phase | 75.08 ± 2.71 |
| 2 | 5 | 2 | 3 | 0 | 0.79 | 27 | Lower Phase | 0 |
| 3 | 4 | 2.6 | 2.4 | 1 | 0.61 | 7 | Lower Phase | 71.15 ± 2.30 |
| 4 | 4 | 3.2 | 1.8 | 2 | 0.59 | 16 | Lower Phase | 33.44 ± 2.54 |
| 5 | 2 | 3.8 | 1.2 | 4 | 1.63 | 46 | Lower Phase | 52.79 ± 2.39 |
| 6 | 1 | 4.4 | 0.6 | 4 | 1.67 | 41 | Lower Phase | 63.61 ± 3.01 |
| 7 | 0 | 5 | 0 | 5 | 1.27 | 16 | Lower Phase | 66.89 ± 2.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Li, T.; Hu, X.; Yang, Y.; Huang, Y.; He, K. A Neglected Issue: Stationary Phase Retention Determination of Classic High-Speed Counter-Current Chromatography Solvent Systems. Separations 2022, 9, 357. https://doi.org/10.3390/separations9110357
Li S, Li T, Hu X, Yang Y, Huang Y, He K. A Neglected Issue: Stationary Phase Retention Determination of Classic High-Speed Counter-Current Chromatography Solvent Systems. Separations. 2022; 9(11):357. https://doi.org/10.3390/separations9110357
Chicago/Turabian StyleLi, Sha, Tiandan Li, Xiaochao Hu, Yong Yang, Yangyi Huang, and Kai He. 2022. "A Neglected Issue: Stationary Phase Retention Determination of Classic High-Speed Counter-Current Chromatography Solvent Systems" Separations 9, no. 11: 357. https://doi.org/10.3390/separations9110357
APA StyleLi, S., Li, T., Hu, X., Yang, Y., Huang, Y., & He, K. (2022). A Neglected Issue: Stationary Phase Retention Determination of Classic High-Speed Counter-Current Chromatography Solvent Systems. Separations, 9(11), 357. https://doi.org/10.3390/separations9110357