Phytochemical and Biological Characterization of the Fractions of the Aqueous and Ethanolic Extracts of Parthenium hysterophorus
Abstract
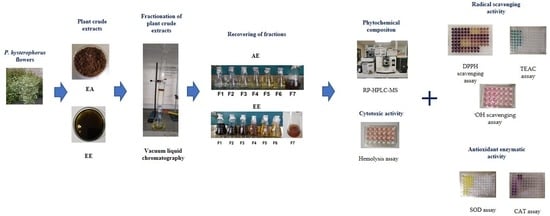
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of the AE and EE Extracts of P. hysterophorus
2.2.1. AE Crude Extract
2.2.2. EE Crude Extract
2.3. Fractionation of the AE and EE Extracts of P. hysterophorus
2.3.1. Selection of Mobile Phase for the Fractionation of the Crude Extracts
2.3.2. Fractionation of the AE and EE Extracts by Vacuum Liquid Chromatography (VLC)
2.4. Phytochemical Identification of the Fractions of the AE and EE Extracts by Reverse-Phase High Performance Liquid Chromatography/Mass Spectrometry (RP-HPLC-MS)
2.5. In Vitro Cytotoxicity Assay
2.6. In Vitro Antioxidant Activity
2.6.1. Radical Scavenging Activity
2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
Hydroxyl (-OH) Radical Scavenging Assay
2.6.2. Antioxidant Enzyme Activity
Superoxide Dismutase (SOD) Activity
Catalase (CAT) Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Selection of Mobile Phases by TLC Test for the Fractionation of the AE and EE Extracts
3.2. Yield Percentages of the Fractions of the AE and EE Extracts
3.3. Phytochemical Composition
3.4. Hemolytic Activity
3.5. Antioxidant Activity
3.5.1. Radical Scavenging Activity
3.5.2. Antioxidant Enzyme Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 22 September 2022).
- Ighodaro, O.M.; Akinloye, O.A. First line defense antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defense grid. Alex. Med. J. 2018, 54, 287–293. [Google Scholar]
- Abubakar, A.R.; Haque, M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J. Pharm. Bioall. Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Jiménez, M.A.; Zugasti-Cruz, A.; Silva-Belmares, S.A.; Ascacio-Valdés, J.A.; Sierra-Rivera, C.A. Phytochemical and biological characerization of aqueous and ethanolic extracts of Parthenium hysterophorus. Pharmacog. J. 2021, 13, 1122–1133. [Google Scholar] [CrossRef]
- Oshiade De Silva, G.; Theekshana-Abeysundara, A.; Weroshana-Aponso, M.M. Extraction methods, qualitative and quantitative techniques for screening of phytochemicals from plants. Am. J. Essent. Oil. 2017, 5, 29–32. [Google Scholar]
- Rao, M.V.; Sengar, A.S.; Sunil, C.K.; Rawson, A. Ultrasonication—A green technology extraction technique for spices: A review. Trends Food Sci. Technol. 2022, 116, 975–991. [Google Scholar] [CrossRef]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2004, 78, 517–520. [Google Scholar] [CrossRef] [Green Version]
- Efferth, T.; Koch, E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr. Drugs Targets. 2011, 12, 122–132. [Google Scholar] [CrossRef]
- Coskun, O. Separation techniques: Chromatography. NCI 2016, 3, 156–160. [Google Scholar]
- Sierra-Rivera, C.A.; Cobos-Puc, L.E.; Rodríguez-Salazar, M.C.; Iliná, A.; Segura-Ceniceros, E.P.; Solís-Salas, L.M.; Silva-Belmares, S.Y. Applied techniques for extraction, purification, and characterization of medicinal plants active compounds. In Natural Products Chemistry, 1st ed.; Volova, T.G., Mahapatra, D.K., Khanna, S., Haghi, A.K., Eds.; CRC Press: Cleveland, OH, USA, 2021; Volume 1, pp. 155–190. [Google Scholar]
- Bagchi, A.; Raha, A.; Mukherjee, P. A complete review on Parthenium hysterophorus Linn. Int. J. Recent. Adv. Pharm. Res. 2016, 6, 42–49. [Google Scholar]
- Ahmad, J.; Bagheri, R.; Bashir, H.; Baig, M.A.; Al-Huqail, A.; Ibrahim, M.M.; Qureshi, M.I. Organ-specific phytochemical profiling and antioxidant analysis of Parthenium hysterophorus L. Biomed. Res. Int. 2018, 1, 9535232. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Mishra, A.; Pandey, A. Antioxidant mediated protective effect of Parthenium hysterophorus against oxidative damage using in vitro models. BMC Complem. Altern. M 2013, 13, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Chashoo, G.; Saxena, A.K.; Pandey, A.K. Parthenium hysterophorus: A probable source of anticancer, antioxidant and anti-HIV agents. Biomed. Res. Int. 2013, 2013, 810734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, S.N.; Paul, D. Antioxidant potentials of Parthenium hysterophorus L. leaf extracts. SRJI 2014, 3, 80–86. [Google Scholar]
- Iqbal, J.; Ali-Khan, A.; Aziz, T.; Ali, W.; Ahmad, S.; Rahman, S.U.; Iqbal, Z.; Dablool, A.S.; Alrunways, M.W.; Almalki, A.A.; et al. Phytochemical investigation, antioxidant properties and in vivo evaluation of the toxic effects of Parthenium hysterophorus. Molecules 2022, 27, 4189. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Bladt, S. Flavonoids drugs including Ginko biloba and Echnaceae species. In Plant Drug Analysis, 2nd ed.; Wagner, H., Bladt, S., Eds.; Springer: Berlin, Germany, 2001; Volume 1, pp. 195–245. [Google Scholar]
- Solís-Salas, L.M.; Sierra-Rivera, C.A.; Cobos-Puc, L.E.; Ascacio-Valdés, J.A.; Silva-Belmares, S.Y. Antibacterial potential by rupture membrane and antioxidant capacity of purified phenolic fractions of Persea americana leaf extract. Antibiotics 2021, 10, 508. [Google Scholar] [CrossRef]
- De León-Medina, J.C.; Sepúlveda-Torre, L.; Morlett-Chávez, J.; Meléndez-Renteira, P.; Zugasti-Cruz, A.; Ascacio-Valdés, J.; Aguilar, C.N. Solid-state fermentation with Aspergillus niger GH1 to enhance polyphenolic content and antioxidative activity of Castilla Rose (Purshia plicata). Plants 2020, 9, 1518. [Google Scholar]
- Zugasti, A.; Rivera, A.L.; Silva, S.Y.; Alfaro, M.A.; Sierra, C.A. Effect of sodium dichloroacetate as single agent or in combination with cisplatin in normal and human cervical cancer cell lines. Trop. J. Pharm. Res. 2020, 19, 467–474. [Google Scholar] [CrossRef]
- Arituluk, Z.C.; Tatli-Çankaya, I.I.T.; Gençler-Özka, N.A.M. Antioxidant activity, total phenolic and flavonoid contents of some Tanacetum L. (Asteraceae) Taxa growing in Turkey. FABAD J. Pharm. Sci. 2016, 41, 17–25. [Google Scholar]
- Ozyurek, M.; Bektasoglu, B.; Guclu, K.; Apak, R. Hydroxyl radical scavenging assay of phenolics and flavonoids with a modified cupric reducing antioxidant capacity (CUPRAC) method using catalase for hydrogen peroxide degradation. Anal. Chim. Acta 2008, 616, 196–206. [Google Scholar] [CrossRef]
- Begum, A.; Rao, K.N.V.; Dutt, R.; Sindhu, G.K.; Fathima, K.U.; Gowthami, G.; Kumar, J.V.; Naveen, B.; Shaffath, S. Phytochemical screening and thin layer chromatography of Indian Asparagus officinalis Linn. Int. J. Adv. Res. 2017, 5, 1520–1528. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-Rodríguez, G.; Plaza, M.; Marina, M.L. High-performance thin-layer chromatography and direct analysis in real time-high resolution mass spectrometry of non-extractable polyphenols from tropical fruit peels. Food Res. Int. 2021, 147, 110455. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Patil, D.; Patil, A. Qualitative HPTLC phytochemical profiling of Careya arborea Roxb. Bark, leaves and seeds. Biotech 2019, 9, 311. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Wightman, E.L. Herbal extracts and phytochemicals: Plant secondary metabolites and the enhancement of human brain function. Adv. Nutr. 2011, 2, 32–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Wang, Z.; Xie, Y.; Hu, H. Antitumor activity and mechanism of costunolide and dehydrocostus lactones: Two natural sesquiterpene lactones from the Asteraceae family. Biomed. Pharm. 2020, 125, 109955. [Google Scholar] [CrossRef]
- Zhang, Z.; Pang, X.; Xuewu, D.; Ji, Z.; Jiang, Y. Role of peroxidase in anthocyanin degradation in litchi fruit pericarp. Food Chem. 2005, 90, 47–52. [Google Scholar] [CrossRef]
- Strassburg, C.; Kalthoff, S. Coffee and gastrointestinal glucoronosyltransferases. In Coffee in Health and Disease Prevention, 1st ed.; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 1, pp. 535–543. [Google Scholar]
- Vinholes, J.; Silva, B.M.; Silva, L.R. Hydroxycinnamic acids (HCAS): Structure, biological properties and health effects. In Advances in Medicine and Biology, 1st ed.; Berhardt, L.V., Ed.; Nova Science Publishers: New York, NY, USA, 2015; Volume 88, pp. 1–33. [Google Scholar]
- Abramovič, H. Antioxidant properties of hydroxycinnamic acid derivatives: A focus on biochemistry, physicochemical parameters, reactive species, and biomolecular interactions. In Coffee in Health and Disease Prevention, 1st ed.; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 1, pp. 843–852. [Google Scholar]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Dipankar, C.R.; Munan, S. Toxicology, phyochemistry, bioactive compounds and pharmacology of Parthenium hysterophorus. J. Med. Plants Stud. 2013, 1, 126–141. [Google Scholar]
- Padma, S.; Deepika, S. Phytochemical screening and in vitro anti-fungal investigation of Parthenium hysterophorus extracts against Alternaria alternate. Int. Res. J. Pharm. 2013, 4, 190–193. [Google Scholar]
- Pagano, M.; Faggio, C. The used of erythrocyte fragility to assess xenobiotic cytotoxicity. Cell Biochem. Funct. 2015, 33, 351–355. [Google Scholar] [CrossRef]
- De Freitas-Araújo, M.M.; Hilário, F.; Villegas, W.; Campaner-Dos Santos, L.; Lourenço Brunetti, I.; Sotomayor, C.E.; Bauab, T.M. Correlation among antioxidant, antimicrobial, hemolytic, and antiproliferative properties of Leiothrix spiralis leaves extract. IJMS 2012, 13, 9260–9277. [Google Scholar] [CrossRef]
- Surendra, T.V.; Roopan, S.M.; Arasu, M.V.; Al-Dhabi, N.A.; Sridharan, M. Phenolic compounds in drumstick peel for the evaluation of antibacterial, hemolytic and photocatalytic activities. J. Photochem. Photobiol. B 2016, 161, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.M.; Santos, M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Flow injection-based methods for fast screening of antioxidant capacity. Talanta 2009, 77, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Marín, D.A.; Guevara-Lara, F.; Rivas-Morales, C.; Verduzco-Martínez, J.A.; Galindo-Rodríguez-S, A.; Sánchez-García, E. Biological activity of Nothoscordum bivalve (L.) Britton and Parthenium incanum Kunth extracts. Indian J. Tradit. Know 2018, 17, 699–706. [Google Scholar]
- Bermúdez-Toledo, D.; Boffill-Cárdenas, M.; Valido-Díaz, A.; Martínez-Montalbán, C.M.; Iglesias-Rodríguez, N. In vitro photohemolytic evaluation of Parthenium hysterophorus L. Medicentro 2012, 16, 43–48. [Google Scholar]
- Hussain, A.; Khan, A.A.; Ali, M.; Zamani, G.T.; Iqbal, Z.; Ullah, Q.; Iqbal, J.; Shahzad, M.; Aziz, T. In Vitro and In Vivo assessment of toxic effects of Parthenium hysterophorus leaves extract. J. Chil. Chem. Soc. 2022, 67, 5484–5489. [Google Scholar] [CrossRef]
- Quynh-Vo, N.N.; Fukushima, E.O.; Muranaka, T. Structural and hemolytic activity relationships of triterpenoid saponins and sapogenins. J. Nat. Med. 2017, 71, 50–58. [Google Scholar]
- Cheok, C.Y.; Karim-Salman, H.A.; Sulaiman, R. Extraction and quantification of saponins: A review. Food Res. J. 2014, 59, 16–40. [Google Scholar] [CrossRef]
- Chua, L.S.; Lau, C.H.; Chew, C.Y.; Salim-Dawood, D.A. Solvent fractionation and acetone precipitation for crude saponins from Eurycoma longifolia extract. Molecules 2019, 24, 1416. [Google Scholar] [CrossRef] [Green Version]
- Quillay-Dávila, M.A.; Arana-Arias, Y.A.; Jaramillo-Jaramillo, C.G.; Cuenca-Buele, S.; Rojas de Astudillo, L.L.; Jaramillo-Alcívar, V. Saponin content and cicatrizing activity of Cecropia peltata and Parthenium hysterophorus. Rev. Cubana Farm. 2017, 51, 1–15. [Google Scholar]
- Apak, R.; Özyürek, M.; Güclu, K.; Çapanoglu, E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Panwar, R.; Sharma, A.K.; Dutt, D.; Pruthi, V. Phenolic acids from Parthenium hysterophorus: Evaluation of bioconversion potential as free radical scavengers and anticancer agents. Adv. Biosci. Biotechnol. 2015, 6, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Pandey, A.K. Phytochemical screening, GC-MS analysis and In vitro antioxidant potential of Parthenium hysterophorus stem sequentially extracted fractions. Chem. Biol. Lett. 2020, 7, 235–241. [Google Scholar]
- Wu, C.; Chen, F.; Wang, X.; Kim, H.J.; He, G.; Haley-Zitlin, V.; Huang, G. Antioxidant constituents in feverfew (Tanacetum parthenium) extract and their chromatographic quantification. Food Chem. 2006, 96, 220–227. [Google Scholar] [CrossRef]
- Khan, R.A.; Ahmed, M.; Khan, M.R.; Yasir, M.; Muhammad, B.; Khan, R. Nutritional investigation and biological activities of Parthenium hysterophorus. AJPP 2011, 5, 2073–2078. [Google Scholar]
- Lee, M.T.; Lin, W.C.; Yu, B.; Lee, T.T. Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals—A review. Asian-Australas J. Anim. Sci. 2017, 30, 299–308. [Google Scholar] [CrossRef]
- Shang, Y.J.; Liu, B.Y.; Zhao, M.M. Details of the antioxidant mechanism of hydroxycinnamic acids. Czech J. Food Sci. 2015, 33, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Azadmanesh, J.; Borgstahl, G.E.O. A review of the catalytic mechanism of human manganese superoxide dismutase. Antioxidants 2018, 7, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margis, R.; Dunand, C.; Teixeira, F.K.; Margis-Pinheiro, M. Glutathione peroxidase family—An evolutionary overview. FEBS J. 2008, 275, 3959–3970. [Google Scholar] [CrossRef]
- Kaushal, J.; Mehandia, S.; Singh, G.; Raina, A.; Arya, S.K. Catalase enzyme: Application in bioremediation and food industry. ISBAB 2018, 16, 192–199. [Google Scholar] [CrossRef]
- Abrahim, N.N.; Kanthimathi, M.S.; Abdul-Aziz, A. Piper betle shows antioxidant activities, inhibits MCF-7 cell proliferation and increases activities of catalase and superoxide dismutase. BMC Complement. Altern. Med. 2012, 12, 220. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.E.; Hwang, H.J.; Ha, J.S.; Jeong, H.S.; Kim, J.H. Screening of medicinal plant extracts for antioxidant activity. Life Sci. 2003, 73, 167–179. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M. Erythrocytes as a biological model for screening of xenobiotic toxicity. Chem.-Biol. Interact. 2018, 279, 73–83. [Google Scholar] [CrossRef]
- Ma, X.; Deng, D.; Chen, W. Inhibitors and activators of SOD, GSH-Px, and CAT. In Enzyme Inhibitors and Activators, 1st ed.; Şentürk, M., Ed.; Intech Open: Manhattan, NY, USA, 2016; Volume 1, pp. 207–224. [Google Scholar]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88–93. [Google Scholar]
- Díaz, A. The structure of catalases. REB 2002, 22, 76–84. [Google Scholar]
- Xu, Y.; Xin, Y.; Diao, Y.; Lu, C.; Fu, J.; Luo, L.; Yin, Z. Synergistic effects of apigenin and paclitaxel on apoptosis of cancer cells. PLoS ONE 2011, 6, e29169. [Google Scholar] [CrossRef] [Green Version]
- Casano, L.M.; Gómez, L.D.; Lascano, H.R.; González, C.A.; Trippi, V.S. Inactivation and degradation of CuZn-SOD by active oxygen species in wheat chloroplasts exposed to photooxidative stress. Plan Cell Physiol. 2000, 38, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Strycharz-Dudziak, M.; Kiełczykowska, M.; Drop, B.; Świątek, L.; Kliszczewska, E.; Musik, I.; Polz-Dacewicz, M. Total antioxidant status (TAS), superoxide dismutase (SOD), and glutathione peroxidase (GPx) in oropharyngeal cancer associated with EBV infection. Oxid. Med. Cell Longev. 2019, 2019, 5832410. [Google Scholar] [CrossRef]



| Number | Combinations of Solvents | Proportion |
|---|---|---|
| 1 | Ethyl acetate–methanol–deionized water | 100:13.5:10 |
| 2 | Ethyl acetate–methanol | 4:6 |
| 3 | Hexane–chloroform–glacial acetic acid | 45:45:1 |
| 4 | Chloroform–methanol–glacial acetic acid | 47.5:47.5:5 |
| 5 | Hexane–ethyl acetate | 75:25 |
| 6 | Dichloromethane–acetone | 9:1 |
| 7 | Dichloromethane–hexane–methanol | 70:25:5 |
| 8 | Hexane–ethyl acetate–methanol | 80:18:2 |
| 9 | Hexane–acetone | 70:30 |
| 10 | Dichlorometane–acetone–ethyl acetate–methanol–deionized water | 70:20:5:3:2 |
| 11 | Acetone–methanol–glacial acetic acid | 70:25:5 |
| 12 | Chloroform–ethyl acetate | 6:4 |
| 13 | Chloroform | 1 |
| 14 | Ethyl acetate–methanol | 6:2 |
| 15 | Chloroform–methanol–deionized water | 9:1:1 |
| 16 | Chloroform–methanol–glacial acetic acid | 47.5:47.5:5 |
| 17 | Hexane–methanol | 9:1 |
| 18 | Dichlorometane–methanol | 9:1 |
| Combinations of Solvents for the AE Crude Extract | |||
| Fraction | Proportion | ||
| EtOAc | MetOH | H2O | |
| 1 | 100 | 0 | 0 |
| 2 | 80 | 20 | 0 |
| 3 | 60 | 40 | 0 |
| 4 | 40 | 60 | 0 |
| 5 | 20 | 80 | 0 |
| 6 | 0 | 100 | 0 |
| 7 | 0 | 0 | 100 |
| Combinations of Solvents for the EE Crude Extract | |||
| Fraction | Proportion | ||
| CHCl3 | MetOH | H2O | |
| 1 | 100 | 0 | 0 |
| 2 | 80 | 20 | 0 |
| 3 | 60 | 40 | 0 |
| 4 | 40 | 60 | 0 |
| 5 | 20 | 80 | 0 |
| 6 | 0 | 100 | 0 |
| 7 | 0 | 0 | 100 |
| Extract | Rf Values | |
|---|---|---|
| AE | EtOAc-MetOH-H2O (100:13.5:10) | |
| 0.10 | 0.50 | |
| 0.20 | 0.80 | |
| 0.30 | 0.87 | |
| 0.42 | 1.00 | |
| EE | CHCl3-MetOH-H2O (9:1:1) | |
| 0.12 | 0.65 | |
| 0.25 | 0.70 | |
| 0.40 | 0.80 | |
| 0.45 | 0.87 | |
| 0.55 | 0.95 | |
| 0.60 | 1.00 | |
| Fractions of AE | Y% | Fractions of EE | Y% |
|---|---|---|---|
| F1 | 0.04% | F1 | 22.92% |
| F2 | 0.13% | F2 | 16.23% |
| F3 | 0.50% | F3 | 16.58% |
| F4 | 1.05% | F4 | 10.80% |
| F5 | 1.35% | F5 | 8.69% |
| F6 | 1.06% | F6 | 6.74% |
| F7 | 27.47% | F7 | 9.48% |
| Rf Values of the Fractions of the AE | Rf Values of the Fractions of the EE | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F1 | F2 | F3 | F4 | F5 | F6 | F7 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1.00 | 0.75 | 0.10 | 0.10 | 0.10 | 0.50 | 0.20 | 0.77 | 0.07 | 0.45 | 0.10 | 0.10 | 1.00 | 1.00 |
| 1.00 | 0.20 | 0.20 | 0.37 | 0.72 | 0.72 | 0.87 | 0.52 | 0.50 | 0.30 | 0.30 | |||
| 0.37 | 0.37 | 0.50 | 1.00 | 1.00 | 1.00 | 0.57 | 0.55 | 0.45 | 1.00 | ||||
| 0.50 | 0.50 | 0.58 | 0.65 | 0.60 | 1.00 | ||||||||
| 0.57 | 0.57 | 0.70 | 0.72 | 0.65 | |||||||||
| 0.72 | 0.72 | 0.77 | 0.77 | 0.75 | |||||||||
| 0.75 | 0.77 | 1.00 | 0.90 | 1.00 | |||||||||
| 0.77 | 1.00 | 1.00 | |||||||||||
| 1.00 | |||||||||||||
| No. | R.T. (min) | Mass | Compound | Family | Fractions of AE | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | |||||
| 1 | 14.003 | 341 | Caffeic acid 4-O-glucoside | Hydroxycinnamic acids | * | * | * | * | |||
| 2 | 14.659 | 353 | 1-Caffeoylquinic acid | * | * | * | * | ||||
| 3 | 16.981 | 352.9 | 3-Caffeoylquinic acid | * | * | ||||||
| 4 | 20.238 | 134.9 | p-Anisaldehyde | Methoxybenzaldehydes | * | ||||||
| 5 | 21.452 | 337.1 | 3-p-Coumaroylquinic acid | Hydroxycinnamic acids | * | ||||||
| 6 | 21.521 | 664.9 | Luteolin 7-O-(2-apiosyl-6-malonyl)-glucoside | Flavones | * | ||||||
| 7 | 24.429 | 340.9 | Caffeic acid 4-O-glucoside (isomer) | Hydroxycinnamic acids | * | ||||||
| 8 | 28.328 | 476.9 | Isorhamnetin 4’-O-glucoside | Methoxyflavonols | * | * | |||||
| 9 | 28.43 | 341 | Tetramethylscutellarein | Methoxyflavones | * | ||||||
| 10 | 30.52 | 514.9 | 1,3-Dicaffeoylquinic acid | Hydroxycinnamic acids | * | ||||||
| 11 | 31.083 | 324.9 | p-Coumaric acid 4-O-glucoside | * | |||||||
| 12 | 31.378 | 461 | Isorhamnetin 3-O-rutinoside | Methoxyflavonols | * | * | * | ||||
| 13 | 31.586 | 284.9 | Scutellarein | Flavones | * | ||||||
| 14 | 32.194 | 460.9 | Luteolin 7-O-glucuronide | * | |||||||
| 15 | 34.505 | 515 | 1,3-Dicaffeoylquinic acid | Hydroxycinnamic acids | * | * | |||||
| 16 | 36.408 | 307 | Bisdemethoxycurcumin | Curcuminoids | * | ||||||
| 17 | 36.461 | 261 | Dihydrocaffeic acid 3-sulfate | Hydroxycinnamic acids | * | * | |||||
| 18 | 37.527 | 344.9 | Rosmanol | Phenolic terpenes | * | * | * | ||||
| No. | R.T. (min) | Mass | Compound | Family | Fractions of EE | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | |||||
| 1 | 13.97 | 340.9 | Caffeic acid 4-O-glucoside | Hydroxycinnamic acids | * | * | * | ||||
| 2 | 16.54 | 343 | Rosmadial | Phenolic terpenes | * | ||||||
| 3 | 17.44 | 352.9 | 1-Caffeoylquinic acid | Hydroxycinnamic acids | * | * | |||||
| 4 | 26.43 | 371.1 | Sinensetin | Methoxyflavones | * | ||||||
| 5 | 30.46 | 515 | 1,3-Dicaffeoylquinic acid | Hydroxycinnamic acids | * | * | * | ||||
| 6 | 30.51 | 446.9 | Quercetin 3-O-rhamnoside | Flavonols | * | * | |||||
| 7 | 30.95 | 664.9 | Luteolin 7-O-(2-apiosyl-6-malonyl)-glucoside | Flavones | * | ||||||
| 8 | 31.21 | 352.9 | 3-Caffeoylquinic acid | Hydroxycinnamic acids | * | * | * | ||||
| 9 | 32.90 | 754.9 | Quercetin 3-O-rhamnosyl-rhamnosyl-glucoside | Flavonols | * | * | |||||
| 10 | 35.8 | 344.9 | Rosmanol | Phenolic terpenes | * | * | * | * | |||
| 11 | 36.90 | 344.9 | Epirosmanol | * | |||||||
| 12 | 37.61 | 592.9 | Apigenin 6,8-di-C-glucoside | Flavones | * | * | * | ||||
| 13 | 40.82 | 801.1 | Spinacetin 3-O-glucosyl-(1->6)-[apiosyl(1->2)]-glucoside | Methoxyflavonols | * | * | * | ||||
| 14 | 44.23 | 329.1 | 3,7-Dimethylquercetin | * | |||||||
| 15 | 46.49 | 785.2 | Pedunculagin II | Ellagitannins | * | * | |||||
| 16 | 49.11 | 301 | Quercetin | Flavonols | * | ||||||
| 17 | 49.15 | 325 | p-Coumaric acid 4-O-glucoside | Hydroxycinnamic acids | * | * | |||||
| 18 | 51.26 | 311 | Caffeoyl tartaric acid | * | |||||||
| Crude Extract | Concentration (µg/mL) | Hemolysis% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | Asc-A (13.2 µg/mL) | Resv (22.8 µg/mL) | ||
| Negative control | 0 | 0 ± 0.40 | ||||||||
| AE | 200 | 3.57 ± 0.10 * | 1.08 ± 0.72 | 1.33 ± 1.62 | 0 ± 0.06 | 0 ± 0.17 | 0.04 ± 0.20 | 0 ± 0.20 | 0.42 ± 0.17 | 0.73 ± 0.40 |
| 400 | 3.33 ± 0.20 * | 5.02 ± 1.53 * | 0 ± 0.49 | 0 ± 0.11 | 0.45 ± 0.38 | 0.77 ± 0.21 | 0 ± 0.23 | |||
| 600 | 4.70 ± 0.11 * | 8.62 ± 1.75 * | 4.14 ± 1.51 * | 0.37 ± 0.15 | 1.42 ± 0.36 | 0.89 ± 0.41 | 0 ± 0.30 | |||
| 800 | 6.27 ± 1.15 * | 12.84 ± 2.55 * | 2.43 ± 0.17 * | 0.37 ± 0.06 | 2.28 ± 0.35 | 0.89 ± 0.25 | 0 ± 0.51 | |||
| EE | 200 | 1.01 ± 0.52 | 100 ± 1.81 * | 0.12 ± 0.35 | 1.48 ± 0.35 | 8.37 ± 0.11 * | 13.43 ± 0.42 * | 0 ± 0.21 | ||
| 400 | 3.55 ± 0.17 * | 100 ± 1.60 | 0.29 ± 0.46 | 1.06 ± 0 | 10.52 ± 0.51 * | 16.77 ± 1.25 * | 4.41 ± 0.97 * | |||
| 600 | 7.18 ± 0.06 * | 100 ± 2.46 * | 0.08 ± 0.75 | 1.44 ± 0.35 | 10.78 ± 0.75 * | 15.19 ± 1.91 * | 8.98 ± 1.35 * | |||
| 800 | 22.13 ± 1.65 * | 100 ± 1.25 * | 0.04 ± 0.20 | 1.40 ± 0.30 | 9.33 ± 1.08 * | 13.17 ± 3.03 * | 9.23 ± 0.55 * | |||
| Crude Extract | Concentration (µg/mL) | DPPH Inhibition% % | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | Asc-A | Resv | ||
| Negative control | 0 | 0 ± 0.40 | ||||||||
| AE | 200 | N/E | N/E | 38.41 ± 0.55 * | 31.45 ± 0.66 * | 20.84 ± 0.77 * | 15.53 ± 0.11 * | 10.39 ± 1.16 * | 57.32 ± 0.65 * | 83.20 ± 0.11 * |
| 400 | 62.90 ± 0.05 * | 58.92 ± 0.49 * | 44.83 ± 0.88 * | 43.72 ± 0.44 * | 35.21 ± 0.77 * | 56.09 ± 0.20 * | 91.94 ± 0.18 * | |||
| 600 | 88.72 ± 0.03 * | 82.25 ± 0.05 * | 76.89 ± 0.99 * | 70.14 ± 1.31 * | 58.43 ± 0.11 * | 55.24 ± 0.30 * | 93.37 ± 0.27 * | |||
| 800 | 91.65 ± 0.06 * | 80.65 ± 0.22 * | 89.16 ± 0.22 * | 84.63 ± 0.33 * | 78.93 ± 1.38 * | 54.69 ± 0.03 * | 94.08 ± 0.07 * | |||
| IC50 (µg/mL) | N/E | N/E | 317.21 | 334.94 | 412.88 | 447.98 | 529.98 | |||
| EE | 200 | 0 ± 1.11 | 0 ± 0.27 | 15.48 ± 3.52 * | 14.07 ± 1.50 * | 17.68 ± 1.20 * | 15.21 ± 1.86 * | 8.22 ± 0.12 * | ||
| 400 | 0 ± 0.21 | 8.43 ± 0.27 * | 59.89 ± 1.11 * | 54.08 ± 0.24 * | 41.91 ± 0.42 * | 36.66 ± 0.12 * | 24.16 ± 1.71 * | |||
| 600 | 0 ± 1.17 | 13.76 ± 2.65 * | 84.90 ± 0.99 * | 79.30 ± 1.23 * | 58.96 ± 0.48 * | 60.19 ± 0.39 * | 36.78 ± 0.36 * | |||
| 800 | 0 ± 0.66 | 11.55 ± 0.51 * | 91.26 ± 0.18 * | 92.55 ± 0.09 * | 77.91 ± 0.69 * | 76.58 ± 0.04 * | 60.19 ± 0.21 * | |||
| IC50 (µg/mL) | - | 2084.70 | 366.30 | 415.86 | 508.40 | 523.71 | 723.73 | |||
| Crude Extract | Concentration (µg/mL) | -OH Inhibition % | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | Asc-A (13.2 µg/mL) | Resv (22.8 µg/mL) | ||
| Negative control | 0 | 0 ± 0.60 | ||||||||
| AE | 200 | N/E | N/E | 61.79 ± 0.70 * | 59.31 ± 0.10 * | 40.26 ± 0.20 * | 38.34 ± 0.30 * | 39.95 ± 0.10 * | 35.70 ± 0.78 * | 11.49 ± 0.40 * |
| 400 | 69.62 ± 0.81 * | 68.35 ± 0.64 * | 40.79 ± 0.42 * | 35.32 ± 0.10 * | 48.25 ± 1.01 * | 39.64 ± 0.39 * | 15.32 ± 0.40 * | |||
| 600 | 70.50 ± 0.90 * | 68.99 ± 0.10 * | 42.88 ± 0.20 * | 41.97 ± 0.90 * | 57.60 ± 0.70 * | 50.20 ± 0.49 * | 12.03 ± 0.93 * | |||
| 800 | 73.99 ± 0.60 * | 67.61 ± 0.30 * | 41.13 ± 0.11 * | 41.57 ± 0.10 * | 64.92 ± 0.42 * | 54.54 ± 0.49 * | 12.90 ± 0.53 * | |||
| EE | 200 | 20.36 ± 0.92 * | 54.33 ± 0.70 * | 21.57 ± 1.41 * | 35.99 ± 0.10 * | 54.17 ± 0.40 * | 52.76 ± 0.80 * | 59.91 ± 0.90 * | ||
| 400 | 24.26 ± 0.42 * | 57.96 ± 0.30 * | 21.67 ± 0.10 * | 39.25 ± 0.70 * | 58.30 ± 0.30 * | 58.60 ± 0.40 * | 64.35 ± 1.10 * | |||
| 600 | 23.79 ± 0.20 * | 59.54 ± 0.46 * | 24.40 ± 0.60 * | 40.22 ± 0.30 * | 62.03 ± 0.80 * | 60.42 ± 0.20 * | 67.07 ± 0.35 * | |||
| 800 | 25.40 ± 0.40 * | 65.59 ± 0.61 * | 27.49 ± 0.42 * | 45.87 ± 0.30 * | 65.15 ± 0.30 * | 64.95 ± 0.50 * | 67.41 ± 1.18 * | |||
| Crude Extract | Concentration (µg/mL) | SOD (U/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | Asc-A (13.2 µg/mL) | Resv (22.8 µg/mL) | ||
| Negative control | 0 | 9.49 ± 2.16 | ||||||||
| AE | 200 | 650.31 ± 6.25 * | 144.16 ± 34.83 * | 191.48 ± 12.88 * | 0 ± 14.75 | 190.54 ± 9.32 * | 203.40 ± 11.27 * | 143.05 ± 13.04 * | 69.77 ± 4.99 * | 53.98 ± 3.46 * |
| 400 | 517.78 ± 69.5 * | 153.60 ± 3.73 * | 189.75 ± 10.43 * | 230.94 ± 75.89 * | 203.22 ± 1.25 * | 233.30 ± 43.77 * | 154.24 ± 0.93 * | |||
| 600 | 547.29 ± 38.04 * | 173.30 ± 8.42 * | 190.42 ± 0 * | 149.68 ± 62.31 * | 216.34 ± 14.77 * | 224.96 ± 11.24 * | 169.51 ± 1.03 * | |||
| 800 | 465.22 ± 80.78 * | 166.64 ± 3.03 * | 207.72 ± 2.57 * | 217.98 ± 27.04 * | 217.01 ± 2.69 * | 242.71 ± 13.84 * | 169.51 ± 1.03 * | |||
| EE | 200 | 0 ± 0.20 | 0.65 ± 4.12 | 7.99 ± 11.66 | 0 ± 0.18 | 0 ± 0.07 | 0 ± 3.96 | 0 ± 4.06 | ||
| 400 | 0 ± 5.17 | 16.35 ± 6.44 | 7.59 ± 2.11 | 0 ± 0.20 | 0 ± 0.21 | 0 ± 0.47 | 0 ± 3.80 | |||
| 600 | 0 ± 1.28 | 1.93 ± 6.40 | 15.43 ± 5.89 | 0 ± 3.72 | 0 ± 0.63 | 0.77 ± 1.45 | 0 ± 2.71 | |||
| 800 | 10.63 ± 8.48 | 0 ± 4.28 | 5.20 ± 2.31 | 0 ± 3.78 | 1.52 ± 8.57 | 0 ± 0.96 | 0 ± 0.51 | |||
| Crude Extract | Concentration (µg/mL) | CAT (nmol/min/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | Asc-A (13.2 µg/mL) | Resv (22.8 µg/mL) | ||
| Negative control | 0 | 69.73 ± 24.10 | ||||||||
| AE | 200 | 118.18 ± 4.99 * | 84.10 ± 1.66 | 71.76 ± 12.46 | 55.31 ± 4.15 | 63.54 ± 20.77 | 51.20 ± 24.93 | 55.90 ± 1.66 | 0 ± 3.30 | 0 ± 0.97 |
| 400 | 146.98 ± 2.49 * | 88.22 ± 2.49 | 84.10 ± 13.30 | 112.90 ± 9.14 * | 77.64 ± 20.71 | 61.78 ± 6.65 | 31.81 ± 0.83 | |||
| 600 | 108.19 ± 9.14 * | 125.82 ± 14.13 * | 102.91 ± 3.32 * | 119.95 ± 4.95 * | 69.41 ± 20.73 | 88.22 ± 5.82 * | 48.26 ± 4.15 | |||
| 800 | 90.57 ± 7.48 * | 372.61 ± 25.76 * | 77.05 ± 11.63 | 109.96 ± 26.59 * | 60.60 ± 31.58 | 95.86 ± 0 * | 55.90 ± 8.31 | |||
| EE | 200 | 51.79 ± 9.14 | 258.03 ± 14.96 * | 0 ± 21.61 | 0 ± 3.32 | 20.06 ± 20.77 | 18.29 ± 18.28 | 114.66 ± 13.30 * | ||
| 400 | 90.57 ± 0.83 * | 260.38 ± 4.99 * | 3.02 ± 4.31 | 0 ± 7.48 | 44.15 ± 0 | 25.34 ± 8.31 | 179.88 ± 9.14 * | |||
| 600 | 70.59 ± 7.48 | 272.72 ± 10.80 * | 0 ± 8.31 | 21.23 ± 12.46 | 11.24 ± 1.66 | 58.84 ± 25.76 | 119.36 ± 1.66 * | |||
| 800 | 73.53 ± 11.63 | 265.08 ± 51.52 * | 0 ± 10.80 | 45.32 ± 48.20 | 17.71 ± 2.41 | 45.91 ± 22.44 | 134.64 ± 3.32 * | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfaro Jiménez, M.A.; Zugasti Cruz, A.; Silva Belmares, S.Y.; Ascacio Valdés, J.A.; Sierra Rivera, C.A. Phytochemical and Biological Characterization of the Fractions of the Aqueous and Ethanolic Extracts of Parthenium hysterophorus. Separations 2022, 9, 359. https://doi.org/10.3390/separations9110359
Alfaro Jiménez MA, Zugasti Cruz A, Silva Belmares SY, Ascacio Valdés JA, Sierra Rivera CA. Phytochemical and Biological Characterization of the Fractions of the Aqueous and Ethanolic Extracts of Parthenium hysterophorus. Separations. 2022; 9(11):359. https://doi.org/10.3390/separations9110359
Chicago/Turabian StyleAlfaro Jiménez, Miguel A., Alejandro Zugasti Cruz, Sonia Y. Silva Belmares, Juan A. Ascacio Valdés, and Crystel A. Sierra Rivera. 2022. "Phytochemical and Biological Characterization of the Fractions of the Aqueous and Ethanolic Extracts of Parthenium hysterophorus" Separations 9, no. 11: 359. https://doi.org/10.3390/separations9110359
APA StyleAlfaro Jiménez, M. A., Zugasti Cruz, A., Silva Belmares, S. Y., Ascacio Valdés, J. A., & Sierra Rivera, C. A. (2022). Phytochemical and Biological Characterization of the Fractions of the Aqueous and Ethanolic Extracts of Parthenium hysterophorus. Separations, 9(11), 359. https://doi.org/10.3390/separations9110359