Copper Catalyst-Supported Modified Magnetic Chitosan for the Synthesis of Novel 2-Arylthio-2,3-dihydroquinazolin-4(1H)-one Derivatives via Chan–Lam Coupling
Abstract
:1. Introduction
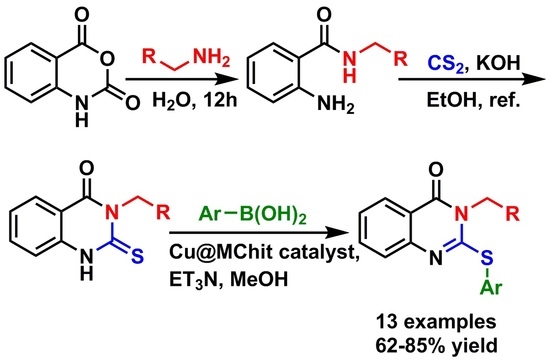
2. Results and Discussion
3. Materials and Methods
3.1. General Remarks
3.2. Synthesis of Cu@MChit Catalyst
3.3. General Procedure for the Synthesis of 2-Amino-N-alkylbenzamide (2)
3.4. General Procedure for the Synthesis of 3-Substituted 2-thioxo-2,3-dihydroquinazolin-4(1H)-one (3)
3.5. General procedure for the Synthesis of 2-Arylthio-2,3-dihydroquinazolin-4(1H)-one Derivatives (4)
3.6. Spectral Data of the Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hameed, A.; Al-Rashida, M.; Uroos, M.; Ali, S.A.; Arshia; Ishtiaq, M.; Khan, K.M. Quinazoline and quinazolinone as important medicinal scaffolds: A comparative patent review (2011–2016). Expert Opin. Ther. Pat. 2018, 28, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, U. Recent developments in the chemistry of quinazolinone alkaloids. Org. Biomol. Chem. 2015, 13, 9336–9352. [Google Scholar] [CrossRef]
- Dohle, W.; Jourdan, F.L.; Menchon, G.; Prota, A.E.; Foster, P.A.; Mannion, P.; Hamel, E.; Thomas, M.P.; Kasprzyk, P.G.; Ferrandis, E. Quinazolinone-based anticancer agents: Synthesis, antiproliferative SAR, antitubulin activity, and tubulin co-crystal structure. J. Med. Chem. 2018, 61, 1031–1044. [Google Scholar] [CrossRef]
- Safakish, M.; Hajimahdi, Z.; Aghasadeghi, M.R.; Vahabpour, R.; Zarghi, A. Design, Synthesis, Molecular Modeling and Anti-HIV Assay of Novel Quinazolinone Incorporated Coumarin Derivatives. Curr. HIV Res. 2020, 18, 41–51. [Google Scholar]
- Sepehri, N.; Iraji, A.; Yavari, A.; Asgari, M.S.; Zamani, S.; Hosseini, S.; Bahadorikhalili, S.; Pirhadi, S.; Larijani, B.; Khoshneviszadeh, M. The natural-based optimization of kojic acid conjugated to different thio-quinazolinones as potential anti-melanogenesis agents with tyrosinase inhibitory activity. Bioorg. Med. Chem. 2021, 36, 116044. [Google Scholar] [CrossRef] [PubMed]
- Yavari, A.; Mohammadi-Khanaposhtani, M.; Moradi, S.; Bahadorikhalili, S.; Pourbagher, R.; Jafari, N.; Faramarzi, M.A.; Zabihi, E.; Mahdavi, M.; Biglar, M. α-Glucosidase and α-amylase inhibition, molecular modeling and pharmacokinetic studies of new quinazolinone-1,2,3-triazole-acetamide derivatives. Med. Chem. Res. 2021, 30, 702–711. [Google Scholar] [CrossRef]
- Kocienski, P. Asymmetric Synthesis of Efinaconazole and Albaconazole. Synfacts 2018, 14, 1221. [Google Scholar]
- Morey, J.; Llinás, P.; Bueno-Costa, A.; León, A.J.; Piña, M.N. Raltitrexed-Modified Gold and Silver Nanoparticles for Targeted Cancer Therapy: Cytotoxicity Behavior In Vitro on A549 and HCT-116 Human Cancer Cells. Materials 2021, 14, 534. [Google Scholar] [CrossRef]
- Peng, J.-B.; Geng, H.-Q.; Wang, W.; Qi, X.; Ying, J.; Wu, X.-F. Palladium-catalyzed four-component carbonylative synthesis of 2,3-disubstituted quinazolin-4(3H)-ones: Convenient methaqualone preparation. J. Catal. 2018, 365, 10–13. [Google Scholar] [CrossRef]
- Maiden, T.; Harrity, J. Recent developments in transition metal catalysis for quinazolinone synthesis. Org. Biomol. Chem. 2016, 14, 8014–8025. [Google Scholar] [CrossRef]
- Rohokale, R.S.; Kshirsagar, U.A. Advanced synthetic strategies for constructing quinazolinone scaffolds. Synthesis 2016, 48, 1253–1268. [Google Scholar]
- Zhang, X.; Luo, C.; Chen, X.; Ma, W.; Li, B.; Lin, Z.; Chen, X.; Li, Y.; Xie, F. Direct synthesis of quinazolinones via the carbon-supported acid-catalyzed cascade reaction of isatoic anhydrides with amides and aldehydes. Tetrahedron Lett. 2021, 66, 152835. [Google Scholar] [CrossRef]
- Verma, E.; Patil, S.; Gajbhiye, A. Convenient Novel Method to Access N-Benzylated Isatoic Anhydride: Reaction Behavior of Isatoic Anhydride with 4-Chlorobenzyl Chloride in the Presence of Bases. ACS Omega 2021, 6, 8346–8355. [Google Scholar] [CrossRef] [PubMed]
- Sajadi, M.S.; Kazemi, E.; Darehkordi, A. Palladium-catalyzed synthesis of novel trifluoromethylated quinazolinone, N-arylquinazoline and N-benzylquinazoline derivatives. Tetrahedron Lett. 2021, 71, 153053. [Google Scholar] [CrossRef]
- Rajput, C.S.; Srivastava, S.; Kumar, A.; Pathak, A. Mukaiyama’s reagent promoted mild protocol for one-pot metal-free synthesis of dihydro quinazolinones. Tetrahedron Lett. 2021, 65, 152791. [Google Scholar] [CrossRef]
- Asgari, M.S.; Bahadorikhalili, S.; Rahimi, R.; Mahdavi, M. Copper Supported onto Magnetic Nanoparticles as an Efficient Catalyst for the Synthesis of Triazolobenzodiazepino [7,1-b]quinazolin-11(9H)-ones via Click N-Arylation Reactions. ChemistrySelect 2021, 6, 1385–1392. [Google Scholar] [CrossRef]
- Sayahi, M.H.; Bahadorikhalili, S.; Saghanezhad, S.J.; Mahdavi, M. Copper (II)-supported polyethylenimine-functionalized magnetic graphene oxide as a catalyst for the green synthesis of 2-arylquinazolin-4(3H)-ones. Res. Chem. Intermed. 2018, 44, 5241–5253. [Google Scholar] [CrossRef]
- Bahadorikhalili, S.; Ashtari, A.; Ma’mani, L.; Ranjbar, P.R.; Mahdavi, M. Copper-supported β-cyclodextrin-functionalized magnetic nanoparticles: Efficient multifunctional catalyst for one-pot ‘green’synthesis of 1,2,3-triazolylquinazolinone derivatives. Appl. Organomet. Chem. 2018, 32, e4212. [Google Scholar] [CrossRef]
- Bahadorikhalili, S.; Ansari, S.; Hamedifar, H.; Ma’mani, L.; Babaei, M.; Eqra, R.; Mahdavi, M. Mo(CO)6-assisted Pd-supported magnetic graphene oxide-catalyzed carbonylation-cyclization as an efficient way for the synthesis of 4(3H)-quinazolinones. Appl. Organomet. Chem. 2019, 33, e4769. [Google Scholar] [CrossRef]
- Bahadorikhalili, S.; Mahdavi, M.; Ma’mani, L.; Shafiee, A.; Mahdavi, H.; Akbarzadeh, T. Palladium functionalized phosphinite polyethyleneimine grafted magnetic silica nanoparticles as an efficient catalyst for the synthesis of isoquinolino [1,2-b] quinazolin-8-ones. New J. Chem. 2018, 42, 5499–5507. [Google Scholar] [CrossRef]
- Sayahi, M.H.; Saghanezhad, S.J.; Bahadorikhalili, S.; Mahdavi, M. CuBr-catalysed one-pot multicomponent synthesis of 3-substituted 2-thioxo-2, 3-dihydroquinazolin-4(1H)-one derivatives. Appl. Organomet. Chem. 2019, 33, e4635. [Google Scholar] [CrossRef] [Green Version]
- Bahadorikhalili, S.; Rahimzadeh, G.; Kianmehr, E.; Ansari, S.; Hamedifar, H.; Mahdavi, M. Facile Non-Transition Metal-Catalyzed Synthesis of 2-Thioxo-2, 3-dihydroquinazolin-4(1H)-one Derivatives via One-Pot Multicomponent Reactions. ChemistrySelect 2019, 4, 100–104. [Google Scholar] [CrossRef]
- Tashrifi, Z.; Rad-Moghadam, K.; Mehrdad, M.; Soheilizad, M.; Larijani, B.; Mahdavi, M. Green synthesis of 2-((2-aryl-3-oxoisoindolin-1-yl) methyl) quinazolin-4(3H)-ones via sequential condensation, sp3 CH bond functionalization and cyclization. Tetrahedron Lett. 2018, 59, 1555–1559. [Google Scholar] [CrossRef]
- Duan, X.; Liu, N.; Liu, K.; Song, Y.; Wang, J.; Mao, X.; Xu, W.; Yang, S.; Li, H.; Ma, J. Copper-promoted Chan-Lam coupling between enaminones and aryl boronic acids. Tetrahedron Lett. 2018, 59, 4187–4190. [Google Scholar] [CrossRef]
- Keesara, S. N-(Pyridin-2-yl) benzamide: Efficient ligand for the nickel catalyzed Chan–Lam cross-coupling reaction. Tetrahedron Lett. 2015, 56, 6685–6688. [Google Scholar] [CrossRef]





| Entry | Solvent | Base | Catalyst/Amount (mg) | Yield % b |
|---|---|---|---|---|
| 1 | DMF | Et3N | Cu@MChit/20 | No reaction |
| 2 | CHCl3 | Et3N | Cu@MChit/20 | 74 |
| 3 | MeOH | Et3N | Cu@MChit/20 | 83 |
| 4 | CH3CN | Et3N | Cu@MChit/20 | No reaction |
| 5 | MeOH | Cs2CO3 | Cu@MChit/20 | No reaction |
| 6 | MeOH | Na2CO3 | Cu@MChit/20 | 63 |
| 7 | MeOH | K2CO3 | Cu@MChit/20 | 54 |
| 8 | MeOH | KOH | Cu@MChit/20 | 35 |
| 9 | MeOH | Et3N | CuI2/20 | No reaction |
| 10 | MeOH | Et3N | CuI/20 | No reaction |
| 11 | MeOH | Et3N | CuCl/20 | 83 |
| 12 | MeOH | Et3N | CuCl2/20 | No reaction |
| 13 | MeOH | Et3N | CuBr/20 | No reaction |
| 14 | MeOH | Et3N | CuOAc/20 | 78 |
| 15 | MeOH | Et3N | No catalyst | No reaction |
| 16 | MeOH | Et3N | Cu@MChit/15 | 55 |
| 17 | MeOH | Et3N | Cu@MChit/30 | 83 |
| 18 | EtOH | Et3N | Cu@MChit/20 | 75 |
 | ||||
| No. | R1 | R2 | Product | Yield % b |
| 4a | Allyl | 4-Cl-phenyl |  | 71 |
| 4b | Allyl | 4-Br-phenyl |  | 80 |
| 4c | Allyl | 4-Me-phenyl |  | 68 |
| 4d | i-propyl | Phenyl |  | 76 |
| 4e | Benzyl | Phenyl |  | 82 |
| 4f | Allyl | 2-Br-phenyl |  | 78 |
| 4g | Phenyl | 2-Me-phenyl |  | 69 |
| 4h | Phenyl | Phenyl |  | 70 |
| 4i | Benzyl | 4-Cl-phenyl |  | 85 |
| 4j | 2-phenylethyl | Phenyl |  | 72 |
| 4k | Benzyl | Phenyl |  | 65 |
| 4l | Allyl | Phenyl |  | 83 |
| 4m | Benzyl | 4-Br-phenyl |  | 62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghasemi, N.; Yavari, A.; Bahadorikhalili, S.; Moazzam, A.; Hosseini, S.; Larijani, B.; Iraji, A.; Moradi, S.; Mahdavi, M. Copper Catalyst-Supported Modified Magnetic Chitosan for the Synthesis of Novel 2-Arylthio-2,3-dihydroquinazolin-4(1H)-one Derivatives via Chan–Lam Coupling. Inorganics 2022, 10, 231. https://doi.org/10.3390/inorganics10120231
Ghasemi N, Yavari A, Bahadorikhalili S, Moazzam A, Hosseini S, Larijani B, Iraji A, Moradi S, Mahdavi M. Copper Catalyst-Supported Modified Magnetic Chitosan for the Synthesis of Novel 2-Arylthio-2,3-dihydroquinazolin-4(1H)-one Derivatives via Chan–Lam Coupling. Inorganics. 2022; 10(12):231. https://doi.org/10.3390/inorganics10120231
Chicago/Turabian StyleGhasemi, Nastaran, Ali Yavari, Saeed Bahadorikhalili, Ali Moazzam, Samanehsadat Hosseini, Bagher Larijani, Aida Iraji, Shahram Moradi, and Mohammad Mahdavi. 2022. "Copper Catalyst-Supported Modified Magnetic Chitosan for the Synthesis of Novel 2-Arylthio-2,3-dihydroquinazolin-4(1H)-one Derivatives via Chan–Lam Coupling" Inorganics 10, no. 12: 231. https://doi.org/10.3390/inorganics10120231
APA StyleGhasemi, N., Yavari, A., Bahadorikhalili, S., Moazzam, A., Hosseini, S., Larijani, B., Iraji, A., Moradi, S., & Mahdavi, M. (2022). Copper Catalyst-Supported Modified Magnetic Chitosan for the Synthesis of Novel 2-Arylthio-2,3-dihydroquinazolin-4(1H)-one Derivatives via Chan–Lam Coupling. Inorganics, 10(12), 231. https://doi.org/10.3390/inorganics10120231