Regioselective One-Pot Synthesis of Novel Functionalized Organoselenium Compound by Bis-Alkoxyselenenylation of Alkenes with Selenium Dibromide and Alcohols
Abstract
:1. Introduction
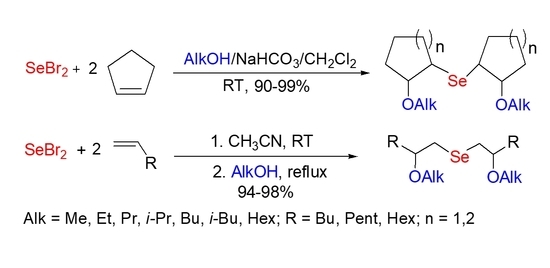
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. The Synthesis of Compound 2
3.3. The Synthesis of Bis(2-alkoxycyclopentyl) Selenides 3–9
3.4. The Synthesis of Bis(2-alkoxycyclohexyl) Selenides 10–13
3.5. The Synthesis of Bis(2-methoxyalkyl) Selenides 14–16
3.6. The Synthesis of Bis(2-alkoxyalkyl) Selenides 20–23
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woollins, J.D.; Laitinen, R.S. (Eds.) Selenium and Tellurium Chemistry. From Small Molecules to Biomolecules and Materials; Springer: Heidelberg/Germany, 2011; p. 334. [Google Scholar]
- Santi, C. (Ed.) Organoselenium Chemistry: Between Synthesis and Biochemistry; Bentham Science Publishers: Sharjah, United Arab Emirates, 2014; p. 563. [Google Scholar]
- Mugesh, G.; du Mont, W.W.; Sies, H. Chemistry of Biologically Important Synthetic Organoselenium Compounds. Chem. Rev. 2001, 101, 2125–2180. [Google Scholar] [CrossRef] [PubMed]
- Lenardao, E.J.; Santi, C.; Sancineto, L. New Frontiers in Organoselenium Compounds; Springer International Publishing AG: Cham, Switzerland, 2018; 189p. [Google Scholar]
- Tiekink, E.R.T. Therapeutic potential of selenium and tellurium compounds: Opportunities yet unrealized. Dalton Trans. 2012, 41, 6390–6395. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B.T. Organoselenium and Organotellurium Compounds: Toxicology and Pharmacology. Chem. Rev. 2004, 104, 6255–6286. [Google Scholar] [CrossRef] [PubMed]
- Ruberte, A.C.; Sanmartin, C.; Aydillo, C.; Sharma, A.K.; Plano, D. Development and Therapeutic Potential of Selenazo Compounds. J. Med. Chem. 2020, 63, 1473–1489. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-C.; Kuhn, H.; Daniliuc, C.-G.; Ivanov, I.; Jones, P.G.; du Mont, W.-W. 5-Selenization of salicylic acid derivatives yielded isoform-specific 5-lipoxygenase inhibitors. Org. Biomol. Chem. 2010, 8, 828–834. [Google Scholar] [CrossRef]
- Potapov, V.A. Organic diselenides, ditellurides, polyselenides and polytellurides. Synthesis and reactions. In Patai’s Chemistry of Functional Groups. Organic Selenium and Tellurium Compounds; Rappoport, Z., Ed.; John Wiley and Sons: Chichester, UK, 2013; Volume 4, pp. 765–843. [Google Scholar]
- Braga, A.L.; Rafique, J. Synthesis of biologically relevant small molecules containing selenium. Part B. Anti-infective and anticancer compounds. In Patai’s Chemistry of Functional Groups. Organic Selenium and Tellurium Compounds; Rappoport, Z., Ed.; John Wiley and Sons: Chichester, UK, 2013; Volume 4, pp. 1053–1117. [Google Scholar]
- Azad, G.K.; Tomar, R.S. Ebselen, a promising antioxidant drug: Mechanisms of action and targets of biological pathways. Mol. Biol. Rep. 2014, 41, 4865–4879. [Google Scholar] [CrossRef]
- Ninomiya, M.; Garud, D.R.; Koketsu, M. Biologically significant selenium-containing heterocycles. Coord. Chem. Rev. 2011, 255, 2968–2990. [Google Scholar] [CrossRef]
- Braverman, S.; Cherkinsky, M.; Kalendar, Y.; Jana, R.; Sprecher, M.; Goldberg, I. Synthesis of water-soluble vinyl selenides and their high glutathione peroxidase (GPx)-like antioxidant activity. Synthesis 2014, 46, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Back, T.G.; Moussa, Z. Remarkable Activity of a Novel Cyclic Seleninate Ester as a Glutathione Peroxidase Mimetic and Its Facile in Situ Generation from Allyl 3-Hydroxypropyl. J. Am. Chem. Soc. 2002, 124, 12104–12105. [Google Scholar] [CrossRef]
- Back, T.G.; Moussa, Z. Diselenides and Allyl Selenides as Glutathione Peroxidase Mimetics. Remarkable Activity of Cyclic Seleninates Produced in Situ by the Oxidation of Allyl ω-Hydroxyalkyl Selenides. J. Am. Chem. Soc. 2003, 125, 13455–13460. [Google Scholar] [CrossRef]
- Back, T.G.; Dyck, B.P. A Novel Camphor-Derived Selenenamide That Acts as a Glutathione Peroxidase Mimetic. J. Am. Chem. Soc. 1997, 119, 2079–2083. [Google Scholar] [CrossRef]
- Santi, C.; Tomassini, C.; Sancineto, L. Organic Diselenides: Versatile Reagents, Precursors, and Intriguing Biologically Active Compounds. Chimia 2017, 71, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Petragnani, N.; Stefani, H.A.; Valduga, C.J. Recent advances in selenocyclofunctionalization reactions. Tetrahedron 2001, 57, 1411–1448. [Google Scholar] [CrossRef]
- Kostić, M.D.; Divac, V.M.; Bugarčić, Z.M. Electrophilic Selenocyclofunctionalization in the Synthesis of Biologically Relevant Molecules. Curr. Org. Chem. 2016, 20, 2606–2619. [Google Scholar] [CrossRef]
- Kostić, M.D.; Divac, V.M.; Bugarčić, Z.M. An introduction to the kinetics of the triethylamine-mediated selenocyclofunctionalization of 4-pentenoic acid. J. Mol. Struct. 2019, 1175, 24–27. [Google Scholar] [CrossRef]
- Nieto, J.; Andres, C.; Perez-Encabo, A. 7-Endo Selenocyclization reactions on chiral 3-prenyl- and 3-cinnamyl-2-hydroxymethylperhydro-1,3-benzoxazine derivatives. A way to enantiopure 1,4-oxazepanes. Org. Biomol. Chem. 2015, 13, 9118–9126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedrosa, R.; Andres, C.; Mendiguchia, P.; Nieto, J. Diastereoselective Synthesis of Enantiopure Morpholines by Electrophilic Selenium-Induced 6-exo Cyclizations on Chiral 3-Allyl-2-hydroxymethylperhydro-1,3-benzoxazine Derivatives. J. Org. Chem. 2006, 71, 8854–8863. [Google Scholar] [CrossRef]
- Stefani, H.A.; Costa, I.M.; Silva, D.D.O.; Menezes, P.H.; Rodrigues, A. Selenocyclofunctionalization of β-ketoamides: Synthesis of substituted dihydrofurans. Phosphorus Sulfur Silicon Relat. Elem. 2001, 172, 395–406. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V.; Belozerova, O.V.; Albanov, A.I.; Yarosh, O.G.; Voronkov, M.G. Synthesis of 3,6-dihalo-4,4-dimethyl-1,4-selenasilafulvenes. Chem. Heterocycl. Compd. 2003, 39, 549–550. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V. New Methods for Preparation of Organoselenium and Organotellurium Compounds from Elemental Chalcogens. Russ. J. Org. Chem. 2003, 39, 1373–1380. [Google Scholar] [CrossRef]
- Abakumov, G.A.; Piskunov, A.V.; Cherkasov, V.K.; Fedushkin, I.L.; Ananikov, V.P.; Eremin, D.B.; Gordeev, E.G.; Beletskaya, I.P.; Averin, A.D.; Bochkarev, M.N.; et al. Organoelement chemistry: Promising growth areas and challenges. Russ. Chem. Rev. 2018, 87, 393–507. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A. Selenium dihalides: New possibilities for the synthesis of selenium-containing heterocycles. Chem. Heterocycl. Comp. 2017, 53, 150–152. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A.; Musalova, M.V.; Amosova, S.V. Stereoselective synthesis of (E,E)-bis(2-halovinyl) selenides and its derivatives based on selenium halides and acetylene. Tetrahedron 2012, 68, 10567–10572. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Musalova, M.V.; Rusakov, Y.Y.; Khabibulina, A.G.; Rusakova, I.L.; Amosova, S.V. Stereoselective synthesis of E-2-halovinyl tellanes, ditellanes and selenides based on tellurium tetrahalides, selenium dihalides and internal alkynes. J. Organomet. Chem. 2018, 867, 300–305. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Amosova, S.V. Reactions of selenium dichloride and dibromide with unsaturated ethers. Annulation of 2,3-dihydro-1,4-oxaselenine to the benzene ring. Tetrahedron Lett. 2011, 52, 4606–4610. [Google Scholar] [CrossRef]
- Amosova, S.V.; Penzik, M.V.; Albanov, A.I.; Potapov, V.A. Addition of selenium dibromide to divinyl sulfide: Spontaneous rearrangement of 2,6-dibromo-1,4-thiaselenane to 5-bromo-2-bromomethyl-1,3-thiaselenolane. Tetrahedron Lett. 2009, 50, 306–308. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V.; Volkova, K.A.; Penzik, M.V.; Albanov, A.I. Reactions of selenium dichloride and dibromide with divinyl selenide: Synthesis of novel selenium heterocycles and rearrangement of 2,6-dihalo-1,4-diselenanes. Tetrahedron Lett. 2010, 51, 89–92. [Google Scholar] [CrossRef]
- Potapov, V.A.; Shagun, V.A.; Penzik, M.V.; Amosova, S.V. Quantum chemical studies of the reaction of selenium dichloride with divinyl sulfide and comparison with experimental results. J. Organomet. Chem. 2010, 695, 1603–1609. [Google Scholar] [CrossRef]
- Potapov, V.A.; Volkova, K.A.; Penzik, M.V.; Albanov, A.I.; Amosova, S.V. Synthesis of 4-Bromo-2-bromomethyl-1,3-diselenolane from Selenium Dibromide and Divinyl Selenide. Russ. J. Gen. Chem. 2008, 78, 1990–1991. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Musalova, M.V.; Amosova, S.V. Recent Advances in Organochalcogen Synthesis Based on Reactions of Chalcogen Halides with Alkynes and Alkenes. Curr. Org. Chem. 2016, 20, 136–145. [Google Scholar] [CrossRef]
- Sarbu, L.G.; Hopf, H.; Jones, P.G.; Birsa, L.M. Selenium halide-induced bridge formation in [2.2]paracyclophanes. Beilstein J. Org. Chem. 2014, 10, 2550–2555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arsenyan, P. A simple method for the preparation of selenopheno[3,2-b] and [2,3-b]thiophenes. Tetrahedron Lett. 2014, 55, 2527–2529. [Google Scholar] [CrossRef]
- Arsenyan, P.; Petrenko, A.; Belyakov, S. Improved conditions for the synthesis and transformations of aminomethyl selenophenothiophenes. Tetrahedron 2015, 71, 2226–2233. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A.; Kurkutov, E.O.; Musalova, M.V.; Khabibulina, A.G.; Amosova, S.V. Regioselective syntheses of bis-(2-haloalkyl) selenides and dihalo[bis-(2-haloalkyl)]-λ4-selanes from selenium dihalides and 1-alkenes, and the methoxyselenenylation reaction. Archivoc 2017, iii, 365–376. [Google Scholar]
- Kurkutov, E.O.; Musalov, M.V.; Potapov, V.A.; Larina, L.I.; Amosova, S.V. Rearrangements in methanolysis of bis(2-bromoalkyl)selenides. Russ. J. Org. Chem. 2016, 52, 186–191. [Google Scholar] [CrossRef]
- Accurso, A.A.; Cho, S.-H.; Amin, A.; Potapov, V.A.; Amosova, S.V.; Finn, M.G. Thia-, Aza-, and Selena[3.3.1]bicyclononane Dichlorides: Rates vs. Internal Nucleophile in Anchimeric Assistance. J. Org. Chem. 2011, 76, 4392–4395. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V.; Abramova, E.V.; Musalov, M.V.; Lyssenko, K.A.; Finn, M.G. 2,6-Dihalo-9-selenabicyclo[3.3.1]nonanes and their complexes with selenium dihalides: Synthesis and structural characterization. N. J. Chem. 2015, 39, 8055–8059. [Google Scholar] [CrossRef]
- Abramova, E.V.; Sterkhova, I.V.; Molokeev, M.S.; Potapov, V.A.; Amosova, S.V. First coordination compounds of SeBr2 with selenium ligands: X-ray structural determination. Mendeleev Commun. 2016, 26, 532–534. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A.; Maylyan, A.A.; Khabibulina, A.G.; Zinchenko, S.V.; Amosova, S.V. Selenium Dihalides Click Chemistry: Highly Efficient Stereoselective Addition to Alkynes and Evaluation of Glutathione Peroxidase-Like Activity of Bis(E-2-halovinyl) Selenides. Molecules 2022, 27, 1050. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A.; Yakimov, V.A.; Musalova, M.V.; Maylyan, A.A.; Zinchenko, S.V.; Amosova, S.V. A Regioselective Synthesis of Novel Functionalized Organochalcogen Compounds by Chalcogenocyclofunctionalization Reactions Based on Chalcogen Halides and Natural Products. Molecules 2021, 26, 3729. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A.; Amosova, S.V. Efficient Synthesis of a New Family of 2,6-Disulfanyl-9-selenabicyclo[3.3.1]nonanes. Molecules 2021, 26, 2849. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Petasi, N.A. Selenium in Natural Products Synthesis; CIS: Philadelphia, PA, USA, 1984; 300p. [Google Scholar]
- Wirth, T. Organoselenium Chemistry: Synthesis and Reactions; Wiley-VCH: Weinheim, Germany, 2012; 462p. [Google Scholar]
- Wirth, T. Organoselenium Chemistry—Modern Developments in Organic Synthesis, Topics in Current Chemistry, 208; Springer: Heidelberg, Germany, 2000; 260p. [Google Scholar]
- Paulmier, C. Selenium Reagents and Intermediates in Organic Synthesis; Pergamon: Oxford, UK, 1986; 463p. [Google Scholar]
- Back, T.G. Organoselenium Chemistry: A Practical Approach; Oxford University Press: Oxford, UK, 1999; 295p. [Google Scholar]
- Tanini, D.; Capperucci, A. Ring opening reactions of heterocycles with selenium and tellurium nucleophiles. N. J. Chem. 2019, 43, 11451–11468. [Google Scholar] [CrossRef] [Green Version]
- Eom, T.; Khan, A. Selenium-Epoxy ‘Click’ Reaction and Se-Alkylation—Efficient Access to Organo-Selenium and Selenonium Compounds. Chemistry 2020, 2, 827–836. [Google Scholar] [CrossRef]
- Eom, T.; Khan, A. Polyselenonium salts: Synthesis through sequential selenium-epoxy ‘click’ chemistry and Se-alkylation. Chem. Commun. 2020, 56, 14271–14274. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Arsenyan, P. Rise of diselenides: Recent advances in the synthesis of heteroarylselenides. Coord. Chem. Rev. 2018, 370, 55–68. [Google Scholar] [CrossRef]
- Patil, D.V.; Hong, Y.T.; Kim, H.Y.; Oh, K. Visible-Light-Induced Three-Component Selenofunctionalization of Alkenes: An Aerobic Selenol Oxidation Approach. Org. Lett. 2022, 24, 8465–8469. [Google Scholar] [CrossRef] [PubMed]
- Tanini, D.; Tiberi, C.; Gellini, C.; Salvi, P.R.; Capperucci, A. A Straightforward Access to Stable β-Functionalized Alkyl Selenols. Adv. Synth. Catal. 2018, 360, 3367–3375. [Google Scholar] [CrossRef]
- Trost, B.M.; Tang, W.; Toste, F.D. Divergent Enantioselective Synthesis of (−)-Galanthamine and (−)-Morphine. J. Am. Chem. Soc. 2005, 127, 14785–14803. [Google Scholar] [CrossRef]
- Shin, H.S.; Jung, Y.G.; Cho, H.K.; Park, Y.G.; Cho, C.G. Total Synthesis of (±)-Lycorine from the Endo-Cycloadduct of 3,5-Dibromo-2-pyrone and (E)-β-Borylstyrene. Org. Lett. 2014, 16, 5718–5720. [Google Scholar] [CrossRef]
- Gao, Y.; Wei, Y.; Ma, D. Synthetic Studies toward Plumisclerin A. Org. Lett. 2019, 21, 1384–1387. [Google Scholar] [CrossRef]
- Wirth, T.; Häuptli, S.; Leuenberger, M. Catalytic asymmetric oxyselenenylation–elimination reactions using chiral selenium compounds. Tetrahedron Asymmetry 1998, 9, 547–550. [Google Scholar] [CrossRef]
- Santi, C.; Fragale, G.; Wirth, T. Synthesis of a new chiral nitrogen containing diselenide as a precursor for selenium electrophiles. Tetrahedron Asymmetry 1998, 9, 3625–3628. [Google Scholar] [CrossRef]
- Tiecco, M.; Testaferri, L.; Marini, F.; Santi, C.; Bagnoli, L.; Temperini, A. Asymmetric oxyselenenylation–deselenenylation reactions of alkenes induced by camphor diselenide and ammonium persulfate. A convenient one-pot synthesis of enantiomerically enriched allylic alcohols and ethers. Tetrahedron Asymmetry 1999, 10, 747–757. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick., G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Adv. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Bruker. SADABS; Bruker AXS Inc.: Madison, WI, USA, 2001. [Google Scholar]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potapov, V.A.; Musalov, M.V.; Khabibulina, A.G.; Maylyan, A.A.; Borodina, T.N.; Zinchenko, S.V.; Amosova, S.V. Regioselective One-Pot Synthesis of Novel Functionalized Organoselenium Compound by Bis-Alkoxyselenenylation of Alkenes with Selenium Dibromide and Alcohols. Inorganics 2022, 10, 239. https://doi.org/10.3390/inorganics10120239
Potapov VA, Musalov MV, Khabibulina AG, Maylyan AA, Borodina TN, Zinchenko SV, Amosova SV. Regioselective One-Pot Synthesis of Novel Functionalized Organoselenium Compound by Bis-Alkoxyselenenylation of Alkenes with Selenium Dibromide and Alcohols. Inorganics. 2022; 10(12):239. https://doi.org/10.3390/inorganics10120239
Chicago/Turabian StylePotapov, Vladimir A., Maxim V. Musalov, Alfiya G. Khabibulina, Arkady A. Maylyan, Tatyana N. Borodina, Sergey V. Zinchenko, and Svetlana V. Amosova. 2022. "Regioselective One-Pot Synthesis of Novel Functionalized Organoselenium Compound by Bis-Alkoxyselenenylation of Alkenes with Selenium Dibromide and Alcohols" Inorganics 10, no. 12: 239. https://doi.org/10.3390/inorganics10120239
APA StylePotapov, V. A., Musalov, M. V., Khabibulina, A. G., Maylyan, A. A., Borodina, T. N., Zinchenko, S. V., & Amosova, S. V. (2022). Regioselective One-Pot Synthesis of Novel Functionalized Organoselenium Compound by Bis-Alkoxyselenenylation of Alkenes with Selenium Dibromide and Alcohols. Inorganics, 10(12), 239. https://doi.org/10.3390/inorganics10120239