Two-Photon Fluorescence in Red and Violet Conjugated Polymer Microspheres
Abstract
:1. Introduction
2. Experimental Methods
2.1. Polymer Synthesis
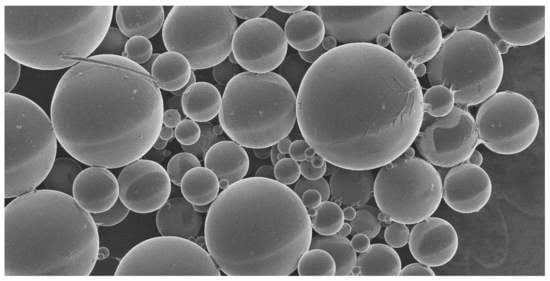
2.2. Microsphere Fabrication
2.3. Optical Measurements
3. Results and Discussion
3.1. One-Photon Absorption and Fluorescence
3.2. Two-Photon Fluorescence
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Helmchen, F.; Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2005, 2, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Yang, M.; Sun, Z.; Gui, R. Ratiometric two-photon fluorescence probes for sensing, imaging and biomedicine applications at living cell and small animal levels. Coord. Chem. Rev. 2021, 446, 214114. [Google Scholar] [CrossRef]
- Wu, C.; Bull, B.; Szymanski, C.; Christensen, K.; McNeill, J. Multicolor conjugated polymer dots for biological fluorescence imaging. ACS Nano 2008, 2, 2415–2423. [Google Scholar] [CrossRef] [PubMed]
- Golovynskyi, S.; Golovynska, I.; Stepanova, L.I.; Datsenko, O.I.; Liu, L.; Qu, J.; Ohulchanskyy, T.Y. Optical windows for head tissues in near-infrared and short-wave infrared regions: Approaching transcranial light applications. J. Biophotonics 2018, 11, e201800141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Nie, J.; Xu, H.; Qiu, Y.; Li, X.; Gu, W.; Tang, G.; Luo, J. Fluorescent microspheres for one-photon and two-photon imaging of mesenchymal stem cells. J. Mater. Chem. B 2017, 5, 7809–7818. [Google Scholar] [CrossRef]
- Gu, M.; Schilders, S.P.; Gan, X. Two-photon fluorescence imaging of microspheres embedded in turbid media. J. Mod. Opt. 2000, 47, 959–965. [Google Scholar] [CrossRef]
- Kachynski, A.V.; Kuzmin, A.N.; Pudavar, H.E.; Kaputa, D.S.; Cartwright, A.N.; Prasad, P.N. Measurement of optical trapping forces by use of the two-photon-excited fluorescence of microspheres. Opt. Lett. 2003, 28, 2288–2290. [Google Scholar] [CrossRef] [Green Version]
- Von Klitzing, W.; Jahier, E.; Long, R.; Lissillour, F.; Lefvre-Seguin, V.; Hare, J.; Raimond, J.-M.; Haroche, S. Very low threshold green lasing in microspheres by up-conversion of IR photons. J. Opt. B Quantum Semiclass. Opt. 2000, 2, 204–206. [Google Scholar] [CrossRef]
- Pang, S.; Beckham, R.E.; Meissner, K.E. Quantum dot-embedded microspheres for remote refractive index sensing. Appl. Phys. Lett. 2008, 92, 221108. [Google Scholar] [CrossRef] [Green Version]
- Perez-Rodriguez, C.; Labrador-Paez, L.; Martin, I.R.; Rios, S. Temperature response of the whispering gallery mode resonances from the green upconversion emission of an Er3+-Yb3+ co-doped microsphere. Laser Phys. Lett. 2015, 12, 046003. [Google Scholar] [CrossRef]
- Soini, J.T.; Soukka, J.M.; Meltola, N.J.; Soini, A.E.; Soini, E.; Hänninen, P.E. Ultra sensitive bioaffinity assay for micro volumes. Single Mol. 2000, 1, 203–206. [Google Scholar] [CrossRef]
- Kurtz, R.; Fricke, M.; Kalb, J.; Tinnefeld, P.; Sauer, M. Application of multiline two-photon microscopy to functional in vivo imaging. J. Neurosci. Methods 2006, 151, 276–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, L.; Gan, X.; Gu, M. Characterization of gradient-index lens-fiber spacing toward applications in two-photon fluorescence endoscopy. Appl. Opt. 2005, 44, 7270–7274. [Google Scholar] [CrossRef] [PubMed]
- Agate, B.; Brown, C.T.A.; Sibbett, W.; Dholakia, K. Femtosecond optical tweezers for in-situ control of two-photon fluorescence. Opt. Express 2004, 12, 3011–3017. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-Y.; Cao, D.; Kang, Y.-F.; Lin, Y.; Cui, R.; Pang, D.-W.; Tang, H.-W. Fluorescence detection of H5N1 virus gene sequences based on optical tweezers with two-photon excitation using a single near infrared nanosecond pulse laser. Anal. Chem. 2016, 88, 4432–4439. [Google Scholar] [CrossRef]
- Wang, W.; Xu, D.; Wei, X.; Chen, K. Magnetic-luminescent YbPO4:Er,Dy microspheres designed for tumor theranostics with synergistic effect of photodynamic therapy and chemotherapy. Int. J. Nanomed. 2014, 9, 4879–4891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Webb, W.W. Measurement of two-photon excitation cross sections of molecular fluorophores with data from 690 to 1050 nm. J. Opt. Soc. Am. B 1996, 13, 481–491. [Google Scholar] [CrossRef]
- De Reguardati, S.; Pahapill, J.; Mikhailov, A.; Stepanenko, Y.; Rebane, A. High-accuracy reference standards for two-photon absorption in the 680–1050 nm wavelength range. Opt. Express 2016, 24, 9053–9066. [Google Scholar] [CrossRef] [Green Version]
- McGehee, M.D.; Heeger, A.J. Semiconducting (conjugated) polymers as materials for solid-state lasers. Adv. Mater. 2000, 12, 1655–1668. [Google Scholar] [CrossRef]
- De Boni, L.; Andrade, A.A.; Corrêa, D.S.; Balogh, D.T.; Zilio, S.C.; Misoguti, L.; Mendonça, C.R. Nonlinear absorption spectrum in MEH-PPV/Chloroform solution: A competition between two-photon and saturated absorption processes. J. Phys. Chem. B 2004, 108, 5221–5224. [Google Scholar] [CrossRef]
- Nagesh, K.; Ramakrishnan, S. Grafting functional handles on to MEHPPV—Possible application for sensing. Synth. Met. 2005, 155, 320–323. [Google Scholar] [CrossRef]
- Wang, S.; Liu, J.; Feng, G.; Ng, L.G.; Liu, B. NIR-II excitable conjugated polymer dots with bright NIR-I emission for deep in vivo two-photon brain imaging through intact skull. Adv. Funct. Mater. 2019, 29, 1808365. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Shao, P.; Qin, J.; Huang, Z.-l. Two-photon absorption property of a conjugated polymer: Influence of solvent and concentration on its property. J. Phys. Chem. B 2010, 114, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Tuncel, D.; Demir, H.V. Conjugated polymer nanoparticles. Nanoscale 2010, 2, 484–494. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, L.; Yang, Q.; Lv, F.; Wang, S. Water-soluble conjugated polymers for imaging, diagnosis, and therapy. Chem. Rev. 2012, 112, 4687–4735. [Google Scholar] [CrossRef]
- Oliveira, S.L.; Corrêa, D.S.; De Boni, L.; Misoguti, L.; Zilio, S.C.; Mendonça, C.R. Two-photon absorption cross-section spectrum of a π-conjugated polymer obtained using the white-light continuum Z-scan technique. Appl. Phys. Lett. 2006, 88, 021911. [Google Scholar] [CrossRef]
- Wu, C.; Szymanski, C.; Cain, Z.; McNeill, J. Conjugated polymer dots for multiphoton fluorescence imaging. J. Am. Chem. Soc. 2007, 129, 12904–12905. [Google Scholar] [CrossRef] [Green Version]
- Braeken, Y.; Cheruku, S.; Ethirajan, A.; Maes, W. Conjugated polymer nanoparticles for bioimaging. Materials 2017, 10, 1420. [Google Scholar] [CrossRef] [Green Version]
- Moses, D. High quantum efficiency luminescence from a conducting polymer in solution: A novel polymer laser dye. Appl. Phys. Lett. 1992, 60, 3215–3216. [Google Scholar] [CrossRef]
- Turnbull, G.A.; Andrew, P.; Barnes, W.L.; Samuel, I.D.W. Operating characteristics of a semiconducting polymer laser pumped by a microchip laser. Appl. Phys. Lett. 2003, 82, 313–315. [Google Scholar] [CrossRef]
- Wasey, J.A.E.; Safonov, A.; Samuel, I.D.W.; Barnes, W.L. Efficiency of radiative emission from thin films of a light-emitting conjugated polymer. Phys. Rev. B 2001, 64, 205201. [Google Scholar] [CrossRef]
- Koynov, K.; Bahtiar, A.; Ahn, T.; Cordeiro, R.M.; Hörhold, H.-H.; Bubeck, C. Molecular weight dependence of chain orientation and optical constants of thin films of the conjugated Polymer MEH-PPV. Macromolecules 2006, 39, 8692–8698. [Google Scholar] [CrossRef]
- Sreelekha, G.; Vidya, G.; Ebin, M.S.; Geetha, K.; Sreeja, S.; Joseph, R.; Pratapan, S.; Radhakrishnan, P.; Vallabhan, C.P.G.; Nampoori, V.P.N. Two photon fluorescence spectra from MEH-PPV/Polystyrene based film waveguides. J. Opt. 2013, 42, 101–105. [Google Scholar] [CrossRef]
- Vattikunta, R.; Annadhasan, M.; Jada, R.; Prasad, M.D.; Mitetelo, N.; Zhdanova, K.; Mamonov, E.; Müllen, K.; Murzina, T.; Chandrasekar, R. Multifunctional chiral π-conjugated polymer microspheres: Production and confinement of NLO signal, detection of circularly polarized light, and display of laser-triggered NLO emission shifts. Adv. Opt. Mater. 2020, 8, 2000431. [Google Scholar] [CrossRef]
- Tabata, K.; Braam, D.; Kushida, S.; Tong, L.; Kuwabara, J.; Kanbara, T.; Beckel, A.; Lorke, A.; Yamamoto, Y. Self-assembled conjugated polymer spheres as fluorescent microresonators. Sci. Rep. 2014, 4, 5902. [Google Scholar] [CrossRef] [Green Version]
- Kushida, S.; Okada, D.; Sasaki, F.; Lin, Z.-H.; Huang, J.-S.; Yamamoto, Y. Low-threshold whispering gallery mode lasing from self-assembled microspheres of single-sort conjugated polymers. Adv. Opt. Mater. 2017, 5, 1700123. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Okada, D.; Kushida, S.; Ngara, Z.S.; Oki, O. Fabrication of polymer microspheres for optical resonator and laser applications. JoVE 2017, 124, e55934. [Google Scholar] [CrossRef]
- Gardner, K.; Aghajamali, M.; Vagin, S.; Pille, J.; Morrish, W.; Veinot, J.G.C.; Rieger, B.; Meldrum, A. Ultrabright fluorescent and lasing microspheres from a conjugated polymer. Adv. Funct. Mater. 2018, 28, 1802759. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Ahn, J.-H.; Shin, D.-C.; Kwon, S.-K. Synthesis and characterization of poly(terphenylenevinylene) derivatives containing alkoxy substituents and (or) phenyl pendant group. Polymer 2004, 45, 2525–2532. [Google Scholar] [CrossRef]
- Matsubara, K.; Ueno, K.; Shibata, Y. Synthesis and structures of nickel halide complexes bearing mono-and bis-coordinated N-heterocyclic carbene ligands, catalyzing Grignard cross-coupling reactions. Organometallics 2006, 25, 3422–3427. [Google Scholar] [CrossRef]
- Chhatre, S.; Ichake, A.; Harpale, K.; Patil, S.; Deshpande, A.; More, M.; Wadgaonkar, P.P. Phenazine-containing poly(phenylenevinylene): A new polymer with impressive field emission properties. J. Polym. Res. 2018, 25, 61. [Google Scholar] [CrossRef]
- Zhang, L.; Mehreen, T.; Liu, X.; Kumar, V.; Vagin, S.; Gardner, K.; Wang, H.; Sun, X.; Wu, S.; Rieger, B.; et al. Wide-Gamut Blended Conjugated Polymer Microspheres. Adv. Opt. Mater. 2021, 9, 2101788. [Google Scholar] [CrossRef]
- Bondarev, D.; Trhlíková, O.; Sedláček, J.; Vohlídal, J. Stability of MEH-PPV: Poly[2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylene]vinylene in solutions exposed to air in the dark and at daylight at laboratory temperature. Polym. Degrad. Stab. 2014, 110, 129–136. [Google Scholar] [CrossRef]
- Ono, T.; Yamada, M.; Suzuki, Y.; Taniguchi, T.; Seki, M. One-step synthesis of spherical/nonspherical polymeric microparticles using non-equilibrium microfluidic droplets. RSC Adv. 2014, 4, 13557–13564. [Google Scholar] [CrossRef]
- Nguyen, T.-Q.; Doan, V.; Schwartz, B.J. Conjugated polymer aggregates in solution: Control of interchain interactions. J. Chem. Phys. 1999, 110, 4068–4078. [Google Scholar] [CrossRef]
- Patterson, G.H.; Piston, D.W. Photobleaching in two-photon excitation microscopy. Biophys. J. 2000, 78, 2159–2162. [Google Scholar] [CrossRef] [Green Version]
- Ibnaouf, K.H. Excimer state of a conjugated polymer (MEH-PPV) in thin films. Opt. Laser Technol. 2013, 48, 401–404. [Google Scholar] [CrossRef]
- Song, L.; Hennink, E.J.; Young, I.T.; Tanke, H.J. Photobleaching kinetics of fluorescein in quantitative fluorescence microscopy. Biophys. J. 1995, 68, 2588–2600. [Google Scholar] [CrossRef]
- Chakraborty, R.; Rothberg, L.J. Role of Aggregates in the Luminescence Decay Dynamics of Conjugated Polymers. J. Phys. Chem. A 2016, 120, 551–555. [Google Scholar] [CrossRef]
- Valandro, S.R.; Jagadesan, P.; Feng, F.; Schanze, K. Aggregation-Enhanced Two-Photon Absorption of Anionic Conjugated Polyelectrolytes. J. Phys. Chem Lett 2020, 11, 8292–8296. [Google Scholar] [CrossRef]
- Zaręba, J.K.; Nyk, M.; Samoć, M. Nonlinear optical pigments. two-photon absorption in crosslinked conjugated polymers and prospects for remote nonlinear optical thermometry. Polymers 2020, 12, 1670. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, M.; Paterson, M.J.; Arnbjerg, J.; Christiansen, O.; Nielsen, C.B.; Jørgensen, M.; Ogilby, P.R. Effects of conjugation length and resonance enhancement on two-photon absorption in phenylene–vinylene oligomers. Phys. Chem. Chem. Phys. 2008, 10, 1177–1191. [Google Scholar] [CrossRef] [PubMed]
- Van Cleuvenbergen, S.; Asselberghs, I.; Vanormelingen, W.; Verbiest, T.; Franz, E.; Clays, K.; Kuzyk, M.G.; Koeckelberghs, G. Record-high hyperpolarizabilities in conjugated polymers. J. Mater. Chem. C 2014, 2, 4533–4538. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Y.; Peng, Z.; Yang, C.; Mu, Q.; Cao, Z.; Ma, J.; Xuan, L. Controlling Morphology and Aggregation in Semiconducting Polymers: The Role of Solvents on Lasing Emission in Poly[2-methoxy-5-(2’-ethyl-hexyloxy)-1,4-phenylene-vinylene]. Materials 2017, 10, 706. [Google Scholar] [CrossRef]
- Nguyen, T.-Q.; Martini, I.B.; Liu, J.; Schwartz, B.J. Controlling interchain Interactions in conjugated polymers: The effects of chain morphology on exciton-exciton annihilation and aggregation in MEH-PPV films. J. Phys. Chem. B 2000, 104, 237–25522. [Google Scholar] [CrossRef] [Green Version]
- Kristoffersen, A.S.; Erga, S.R.; Hamre, B.; Frette, Ø. Testing Fluorescence Lifetime Standards using Two-Photon Excitation and Time-Domain Instrumentation: Rhodamine B, Coumarin 6 and Lucifer Yellow. J. Fluoresc. 2014, 24, 1015–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhi, Y.; Feng, Z.; Mehreen, T.; Liu, X.; Gardner, K.; Li, X.; Guan, B.-O.; Zhang, L.; Vagin, S.I.; Rieger, B.; et al. Two-Photon Fluorescence in Red and Violet Conjugated Polymer Microspheres. Inorganics 2022, 10, 101. https://doi.org/10.3390/inorganics10070101
Zhi Y, Feng Z, Mehreen T, Liu X, Gardner K, Li X, Guan B-O, Zhang L, Vagin SI, Rieger B, et al. Two-Photon Fluorescence in Red and Violet Conjugated Polymer Microspheres. Inorganics. 2022; 10(7):101. https://doi.org/10.3390/inorganics10070101
Chicago/Turabian StyleZhi, Yanyan, Ziwei Feng, Tanisha Mehreen, Xiaoyuan Liu, Kirsty Gardner, Xiangping Li, Bai-Ou Guan, Lijuan Zhang, Sergey I. Vagin, Bernhard Rieger, and et al. 2022. "Two-Photon Fluorescence in Red and Violet Conjugated Polymer Microspheres" Inorganics 10, no. 7: 101. https://doi.org/10.3390/inorganics10070101
APA StyleZhi, Y., Feng, Z., Mehreen, T., Liu, X., Gardner, K., Li, X., Guan, B. -O., Zhang, L., Vagin, S. I., Rieger, B., & Meldrum, A. (2022). Two-Photon Fluorescence in Red and Violet Conjugated Polymer Microspheres. Inorganics, 10(7), 101. https://doi.org/10.3390/inorganics10070101