Temperature-Sensitive Chameleon Luminescent Films Based on PMMA Doped with Europium(III) and Terbium(III) Anisometric Complexes
Abstract
:1. Introduction
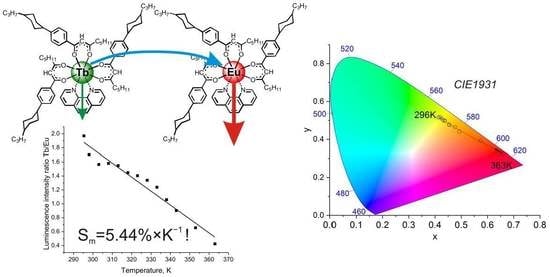
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Characterization Techniques
3.3. Synthesis of Complexes
3.4. Preparation of PMMA–Ln(III) Films
3.5. Calculation of the Quantum Efficiency and Quantum Yield
3.6. Calculation of the Thermal Sensitivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, X.D.; Wolfbeis, O.S.; Meier, R.J. Luminescent probes and sensors for temperature. Chem. Soc. Rev. 2013, 42, 7834–7869. [Google Scholar] [CrossRef] [PubMed]
- Brites, C.D.S.; Millán, A.; Carlos, L.D. Lanthanides in Luminescent Thermometry. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 2016; Volume 49, pp. 339–427. [Google Scholar]
- Brites, C.D.S.; Balabhadra, S.; Carlos, L.D. Lanthanide-Based Thermometers: At the Cutting-Edge of Luminescence Thermometry. Adv. Opt. Mater. 2019, 7, 1801239. [Google Scholar] [CrossRef] [Green Version]
- Moßhammer, M.; Brodersen, K.E.; Kühl, M.; Koren, K. Nanoparticle- and microparticle-based luminescence imaging of chemical species and temperature in aquatic systems: A review. Microchim. Acta 2019, 186, 126. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, E.; Acosta-Mora, P.; Méndez-Ramos, J.; Fischer, S. Optical nanoprobes for biomedical applications: Shining a light on upconverting and near-infrared emitting nanoparticles for imaging, thermal sensing, and photodynamic therapy. J. Mater. Chem. B 2017, 5, 4365–4392. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Lima, P.P.; Silva, N.J.O.; Millán, A.; Amaral, V.S.; Palacio, F.; Carlos, L.D. Thermometry at the nanoscale. Nanoscale 2012, 4, 4799–4829. [Google Scholar] [CrossRef] [Green Version]
- Dramićanin, M.D. Sensing temperature via downshifting emissions of lanthanide-doped metal oxides and salts. A review. Methods Appl. Fluoresc. 2016, 4, 042001. [Google Scholar] [CrossRef] [Green Version]
- Qin, T.; Liu, B.; Zhu, K.; Luo, Z.; Huang, Y.; Pan, C.; Wang, L. Organic fluorescent thermometers: Highlights from 2013 to 2017. TrAC—Trends Anal. Chem. 2018, 102, 259–271. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Kitagawa, Y. Thermo-sensitive luminescence of lanthanide complexes, clusters, coordination polymers and metal-organic frameworks with organic photosensitizers. J. Mater. Chem. C 2019, 7, 7494–7511. [Google Scholar] [CrossRef]
- Dai, Z.; Tian, L.; Song, B.; Ye, Z.; Liu, X.; Yuan, J. Ratiometric Time-Gated Luminescence Probe for Hydrogen Sulfide Based on Lanthanide Complexes. Anal. Chem. 2014, 86, 11883–11889. [Google Scholar] [CrossRef]
- Zhou, J.; Xia, Z.; Bettinelli, M.; Liu, Q. Photoluminescence tuning via energy transfer in Eu-doped Ba2(Gd,Tb)2Si4O13 solid-solution phosphors. RSC Adv. 2015, 6, 2046–2054. [Google Scholar] [CrossRef]
- Bao, G.; Wong, K.-L.; Jin, D.; Tanner, P.A. A stoichiometric terbium-europium dyad molecular thermometer: Energy transfer properties. Light Sci. Appl. 2018, 7, 1–10. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, D.; Cui, Y.; Yang, Y.; Qian, G. A Eu/Tb-mixed MOF for luminescent high-temperature sensing. J. Solid State Chem. 2017, 246, 341–345. [Google Scholar] [CrossRef]
- Lu, H.; Meng, R.; Hao, H.; Bai, Y.; Gao, Y.; Song, Y.; Wang, Y.; Zhang, X. Stark sublevels of Er3+–Yb3+ codoped Gd2(WO4)3 phosphor for enhancing the sensitivity of a luminescent thermometer. RSC Adv. 2016, 6, 57667–57671. [Google Scholar] [CrossRef]
- Lima, N.B.D.; Gonçalves, S.M.C.; Júnior, S.A.; Simas, A.M. A Comprehensive Strategy to Boost the Quantum Yield of Luminescence of Europium Complexes. Sci. Rep. 2013, 3, srep02395. [Google Scholar] [CrossRef] [PubMed]
- Malta, O.L.; Brito, H.F.; Menezes, J.F.S.; Gonçalves e Silva, F.R.; De Mello Donegá, C.; Alves, S. Experimental and theoretical emission quantum yield in the compound Eu(thenoyltrifluoroacetonate)3.2(dibenzyl sulfoxide). Chem. Phys. Lett. 1998, 282, 233–238. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Lima, P.P.; Silva, N.J.O.; Millán, A.; Amaral, V.S.; Palacio, F.; Carlos, L.D. Lanthanide-based luminescent mo-lecular thermometers. New J. Chem. 2011, 35, 1177–1183. [Google Scholar] [CrossRef] [Green Version]
- Binnemans, K. Rare-earth beta-diketonates. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 2005; Volume 35, pp. 107–272. ISBN 9780444520289. [Google Scholar]
- Binnemans, K. Lanthanide-Based Luminescent Hybrid Materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef] [Green Version]
- Felinto Brito, H.; Manoel Loureiro Malta, O.; Claudia França Cunha Felinto, M.; Epaminondas de Sousa Teotonio, E. Lumi-nescence Phenomena Involving Metal Enolates. In PATAI’S Chemistry of Functional Groups; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- De Bettencourt-Dias, A. Lanthanide-based emitting materials in light-emitting diodes. Chem. Rev. 2007, 22, 2229–2241. [Google Scholar] [CrossRef]
- Li, Y.; Bian, Y.; Yan, M.; Thapaliya, P.S.; Johns, D.; Yan, X.; Galipeau, D.; Jiang, J. Mixed (porphyrinato)(phthalocyaninato) rare-earth(III) double-decker complexes for broadband light harvesting organic solar cells. J. Mater. Chem. 2011, 21, 11131–11141. [Google Scholar] [CrossRef]
- Lenaerts, P.; Storms, A.; Mullens, J.; D’Haen, J.; Görller-Walrand, C.; Binnemans, K.; Driesen, K. Thin Films of Highly Luminescent Lanthanide Complexes Covalently Linked to an Organic−Inorganic Hybrid Material via 2-Substituted Imidazo[4,5-f]-1,10-phenanthroline Groups. Chem. Mater. 2005, 17, 5194–5201. [Google Scholar] [CrossRef]
- Li, H.R.; Lin, J.; Zhang, H.J.; Li, H.C.; Fu, L.S.; Meng, Q.G. Novel, covalently bonded hybrid materials of europium (terbium) complexes with silica. Chem. Commun. 2001, 1, 1212–1213. [Google Scholar] [CrossRef]
- He, Y.; Liu, L.; Zhang, Z.; Fu, G.; Lü, X.; Wong, W.-K.; Jones, R.A. A tris-diketonate-Eu(III) complex with the brominated 2,2′-bpy ancillary ligand doped in PMMA for high color-purity red luminescence. Inorg. Chem. Commun. 2016, 64, 13–15. [Google Scholar] [CrossRef]
- George, T.M.; Sajan, M.J.; Gopakumar, N.; Reddy, M.L.P. Bright red luminescence and triboluminescence from PMMA-doped polymer film materials supported by Eu 3+ -triphenylphosphine based β-diketonate and 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene oxide. J. Photochem. Photobiol. A Chem. 2016, 317, 88–99. [Google Scholar] [CrossRef]
- Li, H.; Qi, W.; Sun, H.; Li, P.; Yang, Y.; Wu, L. A novel polymerizable pigment based on surfactant-encapsulated polyoxometalates and their application in polymer coloration. Dye Pigment. 2008, 79, 105–110. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Liu, L.; Yu, C.; Lü, X.; Zhu, X.; Wong, W.-K.; Jones, R.A. Dual-nodal PMMA-supported Eu 3+ -containing metallopolymer with high color-purity red luminescence. Inorg. Chem. Commun. 2015, 60, 51–53. [Google Scholar] [CrossRef]
- Xu, C.-J.; Wan, J.-T.; Li, B.-G. Monochromatic light-emitting copolymer of methyl methacrylate and Eu-complexed 5-acrylamido-1,10-phenanthroline. Dye Pigment. 2013, 98, 493–498. [Google Scholar] [CrossRef]
- Mironov, L.Y.; Evstropiev, S. Temperature-sensitive luminescent photopolymer activated by europium β-diketonate complexes. Opt. Eng. 2019, 58, 027113. [Google Scholar] [CrossRef]
- Knyazev, A.A.; Krupin, A.S.; Molostova, E.Y.; Romanova, K.A.; Galyametdinov, Y.G. Influence of Structural Anisotropy on Mesogenity of Eu(III) Adducts and Optical Properties of Vitrified Films Formed on their Base. Inorg. Chem. 2015, 54, 8987–8993. [Google Scholar] [CrossRef]
- Knyazev, A.A.; Krupin, A.S.; Romanova, K.A.; Galyametdinov, Y.G. Luminescence and energy transfer in poly(N-vinylcarbazole) blends doped by a highly anisometric Eu(III) complex. J. Coord. Chem. 2016, 69, 1473–1483. [Google Scholar] [CrossRef]
- Lapaev, D.V.; Nikiforov, V.G.; Lobkov, V.S.; Knyazev, A.A.; Krupin, A.S.; Galyametdinov, Y.G. New insights into UV laser irradiation effect on luminescent behavior of vitrified films based on mesogenic lanthanide(III) β-diketonate complexes. J. Photochem. Photobiol. A Chem. 2019, 382, 111962. [Google Scholar] [CrossRef]
- Knyazev, A.A.; Karyakin, M.E.; Krupin, A.S.; Romanova, K.A.; Galyametdinov, Y.G. Influence of Eu(III) Complexes Structural Anisotropy on Luminescence of Doped Conjugated Polymer Blends. Inorg. Chem. 2017, 56, 6067–6075. [Google Scholar] [CrossRef] [PubMed]
- Lapaev, D.V.; Nikiforov, V.G.; Knyazev, A.A.; Dzhabarov, V.I.; Lobkov, V.S.; Salikhov, K.M.; Galyametdinov, Y.G. Intramolecular energy transfer in mesogenic europium (III) adduct. Opt. Spectrosc. 2008, 104, 851–857. [Google Scholar] [CrossRef]
- Romanova, K.A.; Freidzon, A.Y.; Bagaturyants, A.A.; Galyametdinov, Y.G. Ab Initio Study of Energy Transfer Pathways in Dinuclear Lanthanide Complex of Europium(III) and Terbium(III) Ions. J. Phys. Chem. A 2014, 118, 11244–11252. [Google Scholar] [CrossRef]
- Knyazev, A.A.; Karyakin, M.E.; Heinrich, B.; Donnio, B.; Galyametdinov, Y.G. A facile approach for the creation of heteroionic lanthanidomesogens-containing uniform films with enhanced luminescence efficiency. Dye Pigment. 2021, 187, 109050. [Google Scholar] [CrossRef]
- Knyazev, A.; Krupin, A.; Gubaidullin, A.; Galyametdinov, Y. Optical and structural characteristics of PMMA films doped with a new anisometric EuIII complex. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2019, 75, 570–577. [Google Scholar] [CrossRef]
- Knyazev, A.A.; Krupin, A.S.; Galyametdinov, Y.G. Luminescence behavior of PMMA films doped with Tb(III) and Eu(III) complexes. J. Lumin. 2021, 242, 118609. [Google Scholar] [CrossRef]
- Liu, J.; Han, X.; Lu, Y.; Wang, S.; Zhao, D.; Li, C. Isostructural Single-and Dual-Lanthanide Metal–Organic Frameworks Based on Substituent-Group-Modifying Tetracarboxylate Ligands for Ratiometric Temperature Sensing. Inorg. Chem. 2021, 60, 4133–4143. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Brites, C.D.S.; Carlos, L.D. Lanthanide Organic Framework Luminescent Thermometers. Chem. Eur. J. 2016, 22, 14782–14795. [Google Scholar] [CrossRef]
- Hatanaka, M.; Hirai, Y.; Kitagawa, Y.; Nakanishi, T.; Hasegawa, Y.; Morokuma, K. Organic linkers control the thermosensitivity of the emission intensities from Tb(iii) and Eu(iii) in a chameleon polymer. Chem. Sci. 2016, 8, 423–429. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Rao, X.; Yu, J.; Cui, Y.; Yang, Y.; Qian, G. Design and Synthesis of an MOF Thermometer with High Sensitivity in the Physiological Temperature Range. Inorg. Chem. 2015, 54, 11193–11199. [Google Scholar] [CrossRef]
- Cadiau, A.; Brites, C.D.S.; Costa, P.M.F.J.; Ferreira, R.A.S.; Rocha, J.; Carlos, L.D. Ratiometric Nanothermometer Based on an Emissive Ln3+-Organic Framework. ACS Nano 2013, 7, 7213–7218. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-H.; Tian, C.-B.; Li, Q.-H.; Du, S.-W. Highly chemical and thermally stable luminescent EuxTb1−xMOF materials for broad-range pH and temperature sensors. J. Mater. Chem. C 2014, 2, 8065–8070. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, B.; Lei, F. Postsynthetic lanthanide functionalization of nanosized metal–organic frameworks for highly sensitive ratiometric luminescent thermometry. Chem. Commun. 2014, 50, 15235–15238. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Song, R.; Yu, J.; Liu, M.; Wang, Z.; Wu, C.; Yang, Y.; Wang, Z.; Chen, B.; Qian, G. Dual-Emitting MOF⊃ Dye Composite for Ratiometric Temperature Sensing. Adv. Mater. 2015, 27, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Piñol, R.; Antorrena, G.; Brites, C.D.S.; Silva, N.J.O.; Murillo, J.L.; Cases, R.; Díez, I.; Palacio, F.; Torras, N.; et al. Luminescent Thermometers: Implementing Thermometry on Silicon Surfaces Functionalized by Lanthanide-Doped Self-Assembled Polymer Monolayers. Adv. Funct. Mater. 2016, 26, 312. [Google Scholar] [CrossRef]
- Xu, M.; Chen, D.; Huang, P.; Wan, Z.; Zhou, Y.; Ji, Z. A dual-functional upconversion core@shell nanostructure for white-light-emission and temperature sensing. J. Mater. Chem. C 2016, 4, 6516–6524. [Google Scholar] [CrossRef]
- Geitenbeek, R.G.; Prins, P.T.; Albrecht, W.; van Blaaderen, A.; Weckhuysen, B.M.; Meijerink, A. NaYF4:Er3+,Yb3+/SiO2 Core/Shell Upconverting Nanocrystals for Luminescence Thermometry up to 900 K. J. Phys. Chem. C 2017, 121, 3503–3510. [Google Scholar] [CrossRef] [Green Version]
- Debasu, M.L.; Ananias, D.; Pastoriza-Santos, I.; Liz-Marzán, L.M.; Rocha, J.; Carlos, L.D. All-in-One Optical Heater-Thermometer Nanoplatform Operative From 300 to 2000 K Based on Er3+ Emission and Blackbody Radiation. Adv. Mater. 2013, 25, 4868–4874. [Google Scholar] [CrossRef]
- Arai, S.; Takeoka, S.; Ishiwata, S.I.; Suzuki, M.; Sato, H. Facilely Fabricated Luminescent Nanoparticle Thermosensor for Real-Time Microthermography in Living Animals. ACS Sens. 2016, 1, 1222–1227. [Google Scholar] [CrossRef]
- Gao, Y.; Huang, F.; Lin, H.; Zhou, J.; Xu, J.; Wang, Y. A Novel Optical Thermometry Strategy Based on Diverse Thermal Response from Two Intervalence Charge Transfer States. Adv. Funct. Mater. 2016, 26, 3139–3145. [Google Scholar] [CrossRef]
- Gharouel, S.; Labrador-Páez, L.; Haro-González, P.; Horchani-Naifer, K.; Férid, M. Fluorescence intensity ratio and lifetime thermometry of praseodymium phosphates for temperature sensing. J. Lumin. 2018, 201, 372–383. [Google Scholar] [CrossRef]
- Borisov, S.M.; Klimant, I. New luminescent oxygen-sensing and temperature-sensing materials based on gadolinium(III) and europium(III) complexes embedded in an acridone–polystyrene conjugate. Anal. Bioanal. Chem. 2012, 404, 2797–2806. [Google Scholar] [CrossRef] [PubMed]
- Borisov, S.M.; Wolfbeis, O.S. Temperature-Sensitive Europium(III) Probes and Their Use for Simultaneous Luminescent Sensing of Temperature and Oxygen. Anal. Chem. 2006, 78, 5094–5101. [Google Scholar] [CrossRef] [PubMed]
- Borisov, S.M.; Klimant, I. Blue LED Excitable Temperature Sensors Based on a New Europium(III) Chelate. J. Fluoresc. 2008, 18, 581–589. [Google Scholar] [CrossRef]
- Knyazev, A.A.; Lobkov, V.S.; Galyametdinov, Y.G. Liquid-crystalline complex of Eu III β-diketonate with 5,5′-di(heptadecyl)-2,2′-bipyridine. Russ. Chem. Bull. 2004, 53, 942–943. [Google Scholar] [CrossRef]
- Dzhabarov, V.I.; Knyazev, A.A.; Nikolaev, V.F.; Galyametdinov, Y.G. Anisotropy of the magnetic susceptibility of mesogeneous lanthanide complexes. Russ. J. Phys. Chem. A 2011, 85, 1450–1453. [Google Scholar] [CrossRef]
- Knyazev, A.A.; Krupin, A.S.; Galyametdinov, Y.G. Anisometric Ln(III) Complexes with Efficient Near-IR Luminescence. Inorganics 2022, 10, 9. [Google Scholar] [CrossRef]
- Knyazev, A.; Karyakin, M.; Galyametdinov, Y. Photostable anisometric lanthanide complexes as promising materials for optical applications. Photonics 2019, 6, 110. [Google Scholar] [CrossRef] [Green Version]
- Bhaumik, M.L. Quenching and Temperature Dependence of Fluorescence in Rare-Earth Chelates. J. Chem. Phys. 1964, 40, 3711–3715. [Google Scholar] [CrossRef]
- Sabbatini, N.; Guardigli, M.; Manet, I.; Ungaro, R.; Casnati, A.; Ziessel, R.; Ulrich, G.; Asfari, Z.; Lehn, J.-M. Lanthanide complexes of encapsulating ligands: Luminescent devices at the molecular level. Pure Appl. Chem. 1995, 67, 135–140. [Google Scholar] [CrossRef]
- Prodi, L.; Maestri, M.; Balzani, V.; Lehn, J.-M.; Roth, C. Luminescence properties of cryptate europium (III) complexes incorporating heterocyclic N-oxide groups. Chem. Phys. Lett. 1991, 180, 45–50. [Google Scholar] [CrossRef]
- Sabbatini, N.; Perathoner, S.; Lattanzi, G.; Dellonte, S.; Balzani, V. Influence of fluoride ions on the absorption and luminescence properties of the [Eu⊂2.2.1]3+ and [Tb⊂2.2.1]3+ cryptates. J. Phys. Chem. 1987, 91, 6136–6139. [Google Scholar] [CrossRef]
- Kropp, J.L.; Windsor, M.W. Luminescence and Energy Transfer in Solutions of Rare-Earth Complexes. I. Enhancement of Fluorescence by Deuterium Substitution. J. Chem. Phys. 1965, 42, 1599–1608. [Google Scholar] [CrossRef]
- Horrocks, W.D.W., Jr.; Sudnick, D.R. Lanthanide ion probes of structure in biology. Laser-induced luminescence decay constants provide a direct measure of the number of metal-coordinated water molecules. J. Am. Chem. Soc. 1979, 101, 334–340. [Google Scholar] [CrossRef]
- Horrocks, W.D.W., Jr.; Sudnick, D.R. Lanthanide ion luminescence probes of the structure of biological macromolecules. Acc. Chem. Res. 1981, 14, 384–392. [Google Scholar] [CrossRef]
- Beeby, A.; Clarkson, I.M.; Dickins, R.S.; Faulkner, S.; Parker, D.; Royle, L.; de Sousa, A.S.; Williams, J.A.G.; Woods, M. Non-radiative deactivation of the excited states of europium, terbium and ytterbium complexes by proximate energy-matched OH, NH and CH oscillators: An improved luminescence method for establishing solution hydration states. J. Chem. Soc. Perkin Trans. 1999, 2, 493–504. [Google Scholar] [CrossRef]
- Kennedy, M.; Aubouy, L.; Jorge, A.B.; Faccini, M.; Noriega, G.; Di Lorenzo, M.; Cocca, M.; Avella, M.; Errico, M.E.; Gentile, G.; et al. Solar cell efficiency enhancement through down-shifting and up-converting layers—The ephocell project; lumi-nescent downshifting quantum yield measurements. Sol. Energy 2010, 830–833. [Google Scholar]
- Collins, S.F.; Baxter, G.W.; Wade, S.; Sun, T.; Grattan, K.T.V.; Zhang, Z.Y.; Palmer, A.W. Comparison of fluorescence-based temperature sensor schemes: Theoretical analysis and experimental validation. J. Appl. Phys. 1998, 84, 4649–4654. [Google Scholar] [CrossRef]








| # | Material | Range, K | Tm, K | Sm, % × K−1 | Optical Parameter | Reference |
|---|---|---|---|---|---|---|
| 1 | 3%Eu5%Tb | 296–363 | 363 | 5.44 | Two intensities | This paper |
| 2 | Eu0.01Tb0.99(hfa)3(dpbp) | 100–450 | 200 | 0.83 | Two intensities | [42] |
| 3 | Eu0.20Tb0.80(bpda) | 303–328 | 328 | 1.39 | Two intensities | [43] |
| 4 | Eu0.01Tb0.99(bdc)1.5·(H2O)2 | 290–320 | 318 | 0.31 | Two intensities | [44] |
| 5 | {Eu0.7Tb0.3(d-cam)(Himdc)2·(H2O)2}3 | 100–450 | 450 | 0.11 | Two intensities | [45] |
| 6 | Eu0.005Tb0.995@In(OH)(bpydc) | 283–333 | 333 | 4.47 | Two intensities | [46] |
| 7 | Eu0.005Tb0.995@Al(OH)(bpydc) | 283–333 | 333 | 3.00 | Two intensities | [46] |
| 8 | Eu2(qptca)(NO3)2(DMF)4]·[CH3CH2OH]3 perylene | 293–353 | 293 | 1.28 | Two intensities | [47] |
| 9 | Ln-DPA (Ln = Eu, Tb) | 293–333 | 293 | 1.5 | Two intensities | [48] |
| 10 | NaGdF4:Yb3+/Ho3+/Ce3+@NaYF4 Yb3+/Tm3+ | 298–393 | 298 | 4.4 | Two intensities | [49] |
| 11 | NaYF4:Yb3+/Er3+@SiO2 | 300–900 | 300 | 1.0 | Two intensities | [50] |
| 12 | Gd2O3:Yb3+/Er3+ | 301–350 | 301 | 1.5 | Two intensities | [51] |
| 13 | Eu3+/RhB-based polymer | 300–310 | 302 | 3.8 | Two intensities | [52] |
| 14 | NaGd(MoO4)2:Tb3+/Pr3+ | 303–483 | 303 | 5.3 | Two intensities | [53] |
| 15 | MPr(PO3)4 (M = Na, Li, K) | 298–363 | 363 | 0.60 | Two intensities | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galyametdinov, Y.G.; Krupin, A.S.; Knyazev, A.A. Temperature-Sensitive Chameleon Luminescent Films Based on PMMA Doped with Europium(III) and Terbium(III) Anisometric Complexes. Inorganics 2022, 10, 94. https://doi.org/10.3390/inorganics10070094
Galyametdinov YG, Krupin AS, Knyazev AA. Temperature-Sensitive Chameleon Luminescent Films Based on PMMA Doped with Europium(III) and Terbium(III) Anisometric Complexes. Inorganics. 2022; 10(7):94. https://doi.org/10.3390/inorganics10070094
Chicago/Turabian StyleGalyametdinov, Yuriy G., Aleksandr S. Krupin, and Andrey A. Knyazev. 2022. "Temperature-Sensitive Chameleon Luminescent Films Based on PMMA Doped with Europium(III) and Terbium(III) Anisometric Complexes" Inorganics 10, no. 7: 94. https://doi.org/10.3390/inorganics10070094
APA StyleGalyametdinov, Y. G., Krupin, A. S., & Knyazev, A. A. (2022). Temperature-Sensitive Chameleon Luminescent Films Based on PMMA Doped with Europium(III) and Terbium(III) Anisometric Complexes. Inorganics, 10(7), 94. https://doi.org/10.3390/inorganics10070094