Synthesis, Characterization, Computational and Biological Activity of Some Schiff Bases and Their Fe, Cu and Zn Complexes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
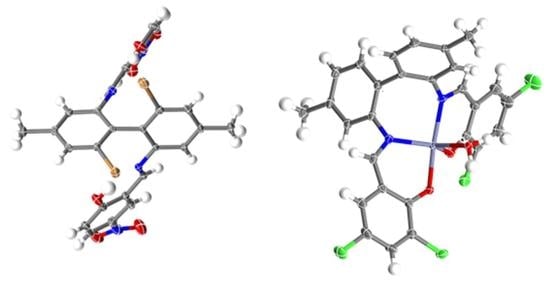
2.2. X-ray Structural Analysis
2.3. Computational Study
2.4. Antimicrobial and Anticancer Assays
3. Experiment
3.1. Materials and Methods
3.2. General Procedure for the Preparation of Ligands (ZH1–ZH4)
3.3. General Procedure for the Synthesis of Cu, Fe and Zn Complexes
3.4. Computational Study
3.5. Antimicrobial Assay
3.6. Anticancer Assay
3.6.1. Cell Culture
3.6.2. Cell Viability Assay (MTT)
3.7. X-ray Structure Determination of ZH4, Z1Zn and Z2Zn
3.7.1. Crystallographic Data for ZH4
3.7.2. Crystallographic Data for Z1Zn
3.7.3. Crystallographic Data for Z2Zn
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Vigato, P.A.; Tamburini, S. The challenge of cyclic and acyclic schiff bases and related derivatives. Coord. Chem. Rev. 2004, 248, 1717–2128. [Google Scholar] [CrossRef]
- Salmon, L.; Bousseksou, A.; Donnadieu, B.; Tuchagues, J.P. Two novel iron (II) materials based on dianionic N4O2 Schiff bases: Structural properties and spin-crossover characteristics in the series [Fe (3-X, 5-NO2-sal-N(1,4,7,10)](X = H, 3-MeO, 3-EtO). Inorg. Chem. 2005, 44, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Keypour, H.; Goudarziafshar, H.; Brisdon, A.K.; Pritchard, R.G.; Rezaeivala, M. New macrocyclic Schiff-base complexes incorporating a phenanthroline unit. Part 2: Template synthesis of some manganese (II) complexes and crystal structure studies. Inorg. Chim. Acta 2008, 361, 1415–1420. [Google Scholar] [CrossRef]
- Abu-Diefa, A.M.; Mohameda, I.M.A. A review on versatile applications of transition metal complexes incorporating Schiff bases. Beni-Seuf Univ. J. Appl. Sci. 2015, 4, 119–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, E.F.; Ferri, D.; Baiker, A.; Van Doorslaer, S.; Schweiger, A. Novel routes to Cu (salicylaldimine) covalently bound to silica: Combined pulse EPR and in situ attenuated total reflection-IR studies of the immobilization. Inorg. Chem. 2003, 42, 2559–2571. [Google Scholar] [CrossRef] [PubMed]
- Fahmi, N.; Masih, I.; Soni, K. Template synthesis, characterization, electrochemical behavior, antimicrobial, nematicidal and pesticidal activities of palladium (II) macrocyclic complexes. J. Macromol. Sci. Pure Appl. Chem. 2015, 52, 548–560. [Google Scholar] [CrossRef]
- Pingaew, R.; Prachayasittikul, S.; Ruchirawat, S. Synthesis, cytotoxic and antimalarial activities of benzoyl thiosemicarbazone analogs of isoquinoline and related compounds. Molecules 2010, 15, 988–996. [Google Scholar] [CrossRef]
- Pavan, F.R.; Maia, P.I.D.S.; Leite, S.R.; Deflon, V.M.; Batista, A.A.; Sato, D.N.; Franzblau, S.G.; Leite, C.Q. Thiosemicarbazones, semicarbazones, dithiocarbazates and hydrazide/hydrazones: Anti–Mycobacterium tuberculosis activity and cytotoxicity. Eur. J. Med. Chem. 2010, 45, 1898–1905. [Google Scholar] [CrossRef]
- Farooqi, S.I.; Arshad, N.; Channar, P.A.; Perveen, F.; Saeed, A.; Larik, F.A.; Javed, A.; Yamin, M. New aryl Schiff bases of thiadiazole derivative of ibuprofen as DNA binders and potential anti-cancer drug candidates. J. Biomol. Struct. Dyn. 2020, 39, 3548–3564. [Google Scholar] [CrossRef]
- Chinnasamy, R.P.; Sundararajan, R.; Govindaraj, S. Synthesis, characterization, and analgesic activity of novel Schiff base of isatin derivatives. J. Adv. Pharm. Technol. Res. 2010, 1, 342. [Google Scholar] [CrossRef] [Green Version]
- Chaubey, A.K.; Pandeya, S.N. Synthesis & anticonvulsant activity (Chemo Shock) of Schiff and Mannich bases of Isatin derivatives with 2-Amino pyridine (mechanism of action). Int. J. Pharmtech Res. 2012, 4, 590–598. [Google Scholar]
- Aboul-Fadl, T.; Mohammed, F.A.H.; Hassan, E.A.S. Synthesis, antitubercular activity and pharmacokinetic studies of some Schiff bases derived from 1-alkylisatin and isonicotinic acid hydrazide (INH). Arch. Pharm. Res. 2003, 26, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Avaji, P.G.; Kumar, C.V.; Patil, S.A.; Shivananda, K.N.; Nagaraju, C. Synthesis, spectral characterization, in-vitro microbiological evaluation and cytotoxic activities of novel macrocyclic bis hydrazone. Eur. J. Med. Chem. 2009, 44, 3552–3559. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Singh, V.; Jain, P.; Tripathi, V. Synthesis, antibacterial, anticancer and molecular docking studies of macrocyclic metal complexes of dihydrazide and diketone. J. Saudi Chem. Soc. 2019, 23, 52–60. [Google Scholar] [CrossRef]
- Liu, J.; Lin, Y.; Liu, M.; Wang, S.; Li, Y.; Liu, X.; Tian, L. Synthesis, structural characterization and cytotoxic activity of triorganotin 5-(salicylideneamino) salicylates. Appl. Organomet. Chem. 2019, 33, e4715. [Google Scholar] [CrossRef]
- Avaji, P.G.; Patil, S.A. Synthesis, spectral characterization and microbiological studies of Co (II), Ni (II) and Cu (II) complexes with some novel 20-membered macrocyclic hydrazino-1,2,4-triazole Schiff bases. J. Enzyme Inhib. Med. Chem. 2009, 24, 140–150. [Google Scholar] [CrossRef]
- Bagihalli, G.B.; Patil, S.A. Synthesis, spectral characterization, in vitro biological and DNA cleavage studies of Co (II), Ni (II), Cu (II), and Zn (II) complexes with 1, 2, 4-triazole Schiff bases. J. Coord. Chem. 2009, 62, 1690–1700. [Google Scholar] [CrossRef]
- Zafar, H.; Kareem, A.; Sherwani, A.; Mohammad, O.; Ansari, M.A.; Khan, H.M.; Khan, T.A. Synthesis and characterization of Schiff base octaazamacrocyclic complexes and their biological studies. J. Photochem. Photobio. B Biol. 2015, 142, 8–19. [Google Scholar] [CrossRef]
- Abd El-Halim, H.F.; Mohamed, G.G.; Anwar, M.N. Antimicrobial and anticancer activities of Schiff base ligand and its transition metal mixed ligand complexes with heterocyclic base. Appl. Organomet. Chem. 2018, 32, e3899. [Google Scholar] [CrossRef]
- Kumar, K.S.; Ganguly, S.; Veerasamy, R.; De Clercq, E. Synthesis, antiviral activity and cytotoxicity evaluation of Schiff bases of some 2-phenyl quinazoline-4 (3) H-ones. Eur. J. Med. Chem. 2010, 45, 5474–5479. [Google Scholar] [CrossRef]
- Nirmal, R.; Meenakshi, K.; Shanmugapandiyan, P.; Prakash, C.R. Synthesis Pharmacological Evaluation of Novel Schiff Base Analogues of 3-(4-amino) Phenylimino) 5-fluoroindolin-2-one. J. Young Pharm. 2010, 2, 162–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ababneh, T.S.; El-khateeb, M.; Tanash, A.K.; AL-Shboul, T.M.; Shammout, M.J.A.; Jazzazi, T.M.; Safa, D.; Samar, T. Synthesis, computational, anticancerous and antiproliferative effects of some copper, manganese and zinc complexes with ligands derived from symmetrical 2,2’-diamino-4,4’-dimethyl-1,1’-biphenyl-salicylaldehyde. Pol. J. Chem. Technol. 2021, 23, 7–15. [Google Scholar] [CrossRef]
- Carter, M.J.; Rillema, D.P.; Basolo, F. Oxygen carrier and redox properties of some neutral cobalt chelates. Axial and in-plane ligand effects. J. Am. Chem. Soc. 1974, 96, 392–400. [Google Scholar] [CrossRef]
- Issa, Y.M.; Sherif, O.E.; Abbas, S.M. Chelation behaviour of Ce (III), Th (IV), and UO2 (VI) with 5,7-dihydroxy-6-formyl-2-methylbenzopyran-4-one Schiff bases. Monatsh. Chem. 1998, 129, 985–998. [Google Scholar] [CrossRef]
- Pandeya, S.N.; Sriram, D.; Nath, G.; De Clercq, E. Synthesis, antibacterial, antifungal and anti-HIV evaluation of Schiff and Mannich bases of isatin derivatives with 3-amino-2-methylmercapto quinazolin-4(3H)-one. Pharm. Acta Helv. 1999, 47, 7–11. [Google Scholar] [CrossRef]
- Upadhyay, K.K.; Kumar, A.; Upadhyay, S.; Mishra, P.C. Synthesis, characterization, structural optimization using density functional theory and superoxide ion scavenging activity of some Schiff bases. J. Mol. Struct. 2008, 873, 5–16. [Google Scholar] [CrossRef]
- Dutta, B.; Some, S.; Ray, J.K. Thermal cyclization of 3-arylamino-3-(2-nitrophenyl)-propenal Schiff base hydrochlorides followed by triethyl phosphite mediated deoxygenation: A facile synthesis of quindolines. Tetrahedron Lett. 2006, 47, 377–379. [Google Scholar] [CrossRef]
- Nishinaga, A.; Yamada, T.; Fujisawa, H.; Ishizaki, K.; Ihara, H.; Matsuura, T. Catalysis of cobalt-Schiff base complexes in oxygenation of alkenes: On the mechanism of ketonization. J. Mol. Cat. 1988, 48, 249–264. [Google Scholar] [CrossRef]
- Petzold, H.; Alrawashdeh, A.I.; Heider, S.; Haufe, L.; Rüffer, T. Synthesis of 6,6′-Bis (dimethylamino)-and 6,6′-Dibromo-Substituted 2,2′-Diphosphanylbiphenyls and Their Palladium Complexes. Eur. J. Inorg. Chem. 2013, 27, 4858–4866. [Google Scholar] [CrossRef]
- Rulev, Y.A.; Gugkaeva, Z.; Maleev, V.I.; North, M.; Belokon, Y.N. Robust bifunctional aluminium–salen catalysts for the preparation of cyclic carbonates from carbon dioxide and epoxides. Beilstien. J. Org. Chem. 2015, 11, 1614–1623. [Google Scholar] [CrossRef] [Green Version]
- Bïlïcï, A.; Kaya, İ.; Doğan, F. Monomer/polymer Schiff base copper (II) complexes for catalytic oxidative polymerization of 2,2′-dihydroxybiphenyl. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 2977–2984. [Google Scholar] [CrossRef]
- Rayati, S.; Rafiee, N.; Wojtczak, A. cis-Dioxo-molybdenum (VI) Schiff base complexes: Synthesis, crystal structure and catalytic performance for homogeneous oxidation of olefins. Inorg. Chim. Acta. 2012, 386, 27–35. [Google Scholar] [CrossRef]
- Reddy, P.M.; Shanker, K.; Srinivas, V.; Krishna, E.R.; Rohini, R.; Srikanth, G.; Ravinder, V. Hydrolysis of Letrozole catalyzed by macrocyclic Rhodium (I) Schiff-base complexes. Spectrochim. Acta Part A Mol. Biomol. Spect. 2015, 139, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Tunçel, M.; Serin, S. Synthesis and characterization of new azo-linked Schiff bases and their cobalt (II), copper (II) and nickel (II) complexes. Trans. Met. Chem. 2006, 31, 805–812. [Google Scholar] [CrossRef]
- Rathi, P.; Singh, D.P. Synthesis, antimicrobial, antioxidant, and molecular docking studies of thiophene based macrocyclic Schiff base complexes. J. Mol. Struct. 2015, 1100, 208–214. [Google Scholar] [CrossRef]
- Boghaei, D.M.; Askarizadeh, E.; Bezaatpour, A. Molecular and biomolecular spectroscopy. Spectrochim. Acta Part A 2008, 69, 624–628. [Google Scholar] [CrossRef]
- Abbaspour, A.; Esmaeilbeig, A.R.; Jarrahpour, A.A.; Khajeh, B.; Kia, R. Aluminium (III)-selective electrode based on a newly synthesized tetradentate Schiff base. Talanta 2002, 58, 397–403. [Google Scholar] [CrossRef]
- Mahajan, R.K.; Kaur, I.; Kumar, M. Silver ion-selective electrodes employing Schiff base p-tert-butyl calix [4] arene derivatives as neutral carriers. Sens. Actuators B Chem. 2003, 91, 26–31. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Mahmood, K.; Wajid, A.; Maah, M.J.; Yusoff, I. Synthesis, characterization, and biological activity of Schiff bases. In Proceedings of the 2011 International Conference on Chemistry and Chemical Process, Bangkok, Thailand, 7–9 May 2011; IACSIT Press: Singapore, 2011; Volume 10, p. 185. [Google Scholar]
- Deivanayagam, P.; Bhoopathy, R.P.; Thanikaikarasan, S. Synthesis, characterization, antimicrobial, analgesic and CNS studies of Schiff base Cu(II) complex derived from 4-choro-o-phenylene diamine. Int. J. Adv. Chem. 2014, 2, 166–170. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, H.; Paul, N.; Rahman, M.L. Catalytic activities of Schiff base aquocomplexes of copper (II) towards hydrolysis of amino acid esters. Trans. Met. Chem. 1994, 19, 524–526. [Google Scholar] [CrossRef]
- El-Hendawy, A.M.; Alkubaisi, A.H.; El-Kourashy, A.E.G.; Shanab, M.M. Ruthenium (II) Complexes of O, N-donor Schiff base ligands and their use as catalytic organic oxidants. Polyhedron 1993, 12, 2343–2350. [Google Scholar] [CrossRef]
- Ababneh, T.S.; Al-Shboul, T.M.; Jazzazi, T.M.; Alomari, M.I.; Görls, H.; Westerhausen, M. Crystallographic and computational study of the structure of copper(II) 2,2′-bis(2-oxidobenzylideneamino)-4,4′-dimethyl-1,1′-biphenyl. Trans. Met. Chem. 2020, 45, 435–442. [Google Scholar] [CrossRef]
- Al-Ebaisat, H.S.; Ababneh, T.S.; Al-Shboul, T.M.; Jazzazi, T.M. Synthesis, characterization and antifungal activity of some substituted 4-thiazolidinone derivatives. J. Pure App. Chem. Res. 2015, 5, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Jazzazi, T.M.A.; Ababneh, T.S.; Abboushi, E.K. Zinc(II) complexes of symmetrical tetradentate Schiff base ligands derived from 2,2’-diamino-6,6’-dibromo-4,4’-dimethyl-1,1’-biphenyl-salicylaldehyde: Synthesis, characterization and computational study. Jord. J. Chem. 2019, 14, 81–87. [Google Scholar]
- Al-Shboul, T.M.; Ziemann, S.; Görls, H.; Krieck, S.; Westerhausen, M. Substituted 2,2′-Bis (2-oxidobenzylideneamino)-4,4′-dimethyl-1,1′-biphenyl Complexes of Zinc. Z. Anorg. Allg. Chem. 2019, 645, 292–300. [Google Scholar] [CrossRef]
- Al-Shboul, T.M.; Ziemann, S.; Görls, H.; Jazzazi, T.M.; Krieck, S.; Westerhausen, M. Synthesis of dipotassium 2,2′-Bis (2-oxidobenzylideneamino)-4,4′-dimethyl-1,1′-biphenyl drivatives and use as ligand transfer reagent. Eur. J. Inorg. Chem. 2018, 14, 1563–1570. [Google Scholar] [CrossRef]
- Daoud, S.; Thiab, S.; Jazzazi, T.M.A.; Alshboul, T.M.A.; Ullah, S. Evaluation and molecular modelling of bis-Schiff base derivatives as potential leads for management of diabetes mellitus. Acta Pharm. 2022, 72, 449–458. [Google Scholar] [CrossRef]
- Thaker, B.; Surati, K.; Oswal, S.; Jadeja, R.; Gupta, V. Synthesis, spectral, thermal and crystallographic investigations on oxovanadium(IV) and manganese(III) complexes derived from heterocyclic β-diketone and 2-amino ethanol. Struct. Chem. 2007, 18, 295–310. [Google Scholar] [CrossRef]
- Miessler, G.; Tarr, D. Inorganic Chemistry, 3rd ed.; Pearson Prentice-Hall: Northfield, MN, USA, 2005. [Google Scholar]
- Agarwal, U.; Singh, N.P.; Kumar, A.; Kumar, K. Synthesis, Spectral Study and Antibacterial Activity of Asymmetrical Tetradentate Schiff Base Complexes. Rasayan J. Chem. 2020, 13, 1685–1691. [Google Scholar] [CrossRef]
- Ejidike, I.P.; Ajibade, P.A. Synthesis, characterization, antioxidant, and antibacterial studies of some metal (II) complexes of tetradentate schiff base ligand:(4E)-4-[(2-(E)-[1-(2, 4-dihydroxyphenyl) ethylidene] aminoethyl) imino] pentan-2-one. Bioinorg. Chem. Appl. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ran, X.; Wang, L.; Lin, Y.; Hao, J.; Cao, D. Syntheses, characterization and biological studies of zinc (II), copper (II) and cobalt (II) complexes with Schiff base ligand derived from 2-hydroxy-1-naphthaldehyde and selenomethionine. Appl. Organomet. Chem. 2010, 24, 741–747. [Google Scholar] [CrossRef]
- Iftikhar, B.; Javed, K.; Khan, M.S.U.; Akhter, Z.; Mirza, B.; Mckee, V. Synthesis, characterization and biological assay of Salicylaldehyde Schiff base Cu (II) complexes and their precursors. J. Mol. Struct. 2018, 1155, 337–348. [Google Scholar] [CrossRef]
- Reshma, R.; Selwin Joseyphus, R.; Arish, D.; Reshmi Jaya, R.J.; Johnson, J. Tridentate imidazole-based Schiff base metal complexes: Molecular docking, structural and biological studies. J. Biomol. Struct. 2021, 40, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Osowole, A.A.; Ott, I.; Ogunlana, O.M. Synthesis, spectroscopic, anticancer, and antimicrobial properties of some metal (II) complexes of (substituted) nitrophenol Schiff base. Int. J. Inorg. Chem. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 9–18. [Google Scholar]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen and sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2’-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Al-Shboul, T.M.A.; Görls, H.; Westerhausen, M. Calcium-mediated hydrophosphination of diphenylethyne and diphenylbutadiyne as well as crystal structure of 1,4-diphenyl-1,4-bis(diphenylphosphanyl)buta-1,3-diene. Inorg. Chem. Commun. 2008, 11, 1419–1421. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. IV. A new dynamical correlation functional and implications for exact-exchange mixing. J. Chem. Phys. 1996, 104, 1040–1046. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colic-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. 1988, B 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Spartan, version 18; Software for Computational Chemistry; Wavefunction. Inc.: Irvine, CA, USA, 2018.
- Kassim, A.; Omuse, G.; Premji, Z.; Revathi, G. Comparison of Clinical Laboratory Standards Institute and European Committee on Antimicrobial Susceptibility Testing guidelines for the interpretation of antibiotic susceptibility at a university teaching hospital in Nairobi, Kenya: A cross-sectional study. Ann. Clin. Microbiol. 2016, 15, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacLowry, J.D.; Jaqua, M.J.; Selepak, S.T. Detailed methodology and implementation of a semiautomated serial dilution microtechnique for antimicrobial susceptibility testing. Appl. Microbio. 1970, 20, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXS-2014, Program for Structure Solution; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Sheldrick, G.M. SHELXL-2014, Program for Structure Refinement; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]







| Bond (Å) | Calc. | Exp. | Angle (°) | Calc. | Exp. |
|---|---|---|---|---|---|
| C1–C15 | 1.492 | 1.498 | O-N-O | 124.53, 124.53 | 122.95, 123.04 |
| C=N | 1.290, 1.290 | 1.265, 1.275 | C-O-H | 107.92, 107.92 | 100.31, 106.76 |
| C-O | 1.332, 1.332 | 1.325, 1.320 | C-N=C | 121.04, 121.03 | 121.20, 120.86 |
| C-Br | 1.918, 1.918 | 1.896, 1.893 | C-C=N | 121.89, 121.90 | 120.41, 120.38 |
| C-N(imine) | 1.408, 1.408 | 1.424, 1.427 | Br-C-CH | 117.56, 117.56 | 118.09, 118.30 |
| C-N(nitro) | 1.461, 1.461 | 1.453, 1.451 | Br-C-C | 120.05, 120.05 | 119.28, 119.05 |
| C-CH3 | 1.510, 1.510 | 1.515, 1.509 | |||
| N···H | 1.742, 1.742 | 1.804, 1.896 |
| Tested Compounds (10 mg mL−1) Inhibition Zone (mm) | Micrococcus luteus (ATCC 934) | Staphylococcus aureus (ATCC 29213) | Escherichia coli (ATCC 25922) |
|---|---|---|---|
| ZH1 | - | - | - |
| ZH2 | 10 | 12 | - |
| ZH3 | 14 | 8 | - |
| ZH4 | 36 | 22 | - |
| [Z1Zn] | - | - | - |
| [Z2Zn] | 15 | 21 | - |
| [Z3Zn] | 25 | 18 | - |
| [Z4Zn] | 15 | 25 | - |
| [Z1Fe] | - | - | - |
| [Z2Fe] | - | 14 | - |
| [Z3Fe] | - | - | - |
| [Z4Fe] | - | 20 | - |
| [Z1Cu] | - | - | 10 |
| [Z2Cu] | - | - | - |
| [Z3Cu] | - | - | 20 |
| [Z4Cu] | - | 11 | - |
| Amoxicillin | 25 | 35 | 10 |
| Cell Lines | ZH1 | ZH2 | ZH3 | ZH4 | Z1Zn | Z1Cu | Z2Zn | Z2Fe | Z2Cu | Z3Zn | Z3Cu | Z4Zn | Z4Fe | Z4Cu | Doxorubicin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A549 | 25.2 | 320.3 | 41.0 | 62.17 | 224.9 | 43.7 | 81.2 | 118.1 | 4.0 | 199.4 | 130.0 | 25.2 | 68.6 | 34.7 | 0.15 |
| MCF7 | 20.5 | 231.7 | 77.5 | Not covered | 17.3 | 10.4 | 25.2 | 41.4 | 1.9 | 17.7 | 172.0 | 14.1 | 36.5 | 19.2 | 0.03 |
| Fibroblasts | 46.8 | 291.8 | 71.1 | 69.2 | 68.2 | 8.5 | 68.7 | 134.1 | 1.5 | 27.6 | 29.2 | 28.3 | 16.7 | 10.4 | 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Shboul, T.M.A.; El-khateeb, M.; Obeidat, Z.H.; Ababneh, T.S.; Al-Tarawneh, S.S.; Al Zoubi, M.S.; Alshaer, W.; Abu Seni, A.; Qasem, T.; Moriyama, H.; et al. Synthesis, Characterization, Computational and Biological Activity of Some Schiff Bases and Their Fe, Cu and Zn Complexes. Inorganics 2022, 10, 112. https://doi.org/10.3390/inorganics10080112
Al-Shboul TMA, El-khateeb M, Obeidat ZH, Ababneh TS, Al-Tarawneh SS, Al Zoubi MS, Alshaer W, Abu Seni A, Qasem T, Moriyama H, et al. Synthesis, Characterization, Computational and Biological Activity of Some Schiff Bases and Their Fe, Cu and Zn Complexes. Inorganics. 2022; 10(8):112. https://doi.org/10.3390/inorganics10080112
Chicago/Turabian StyleAl-Shboul, Tareq M. A., Mohammad El-khateeb, Zaid H. Obeidat, Taher S. Ababneh, Suha S. Al-Tarawneh, Mazhar S. Al Zoubi, Walhan Alshaer, Anas Abu Seni, Taqwa Qasem, Hayato Moriyama, and et al. 2022. "Synthesis, Characterization, Computational and Biological Activity of Some Schiff Bases and Their Fe, Cu and Zn Complexes" Inorganics 10, no. 8: 112. https://doi.org/10.3390/inorganics10080112
APA StyleAl-Shboul, T. M. A., El-khateeb, M., Obeidat, Z. H., Ababneh, T. S., Al-Tarawneh, S. S., Al Zoubi, M. S., Alshaer, W., Abu Seni, A., Qasem, T., Moriyama, H., Yoshida, Y., Kitagawa, H., & Jazzazi, T. M. A. (2022). Synthesis, Characterization, Computational and Biological Activity of Some Schiff Bases and Their Fe, Cu and Zn Complexes. Inorganics, 10(8), 112. https://doi.org/10.3390/inorganics10080112