A Manganese(II) 3D Metal–Organic Framework with Siloxane-Spaced Dicarboxylic Ligand: Synthesis, Structure, and Properties
Abstract
:1. Introduction
2. Results
2.1. FTIR Spectroscopy
2.2. X-ray Crystallography
2.3. Hydrophobic Behaviour
2.4. Thermal and Dielectric Behaviors
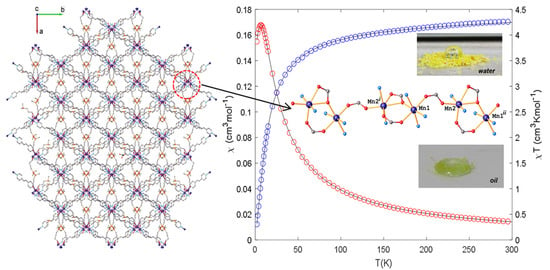
2.5. Magnetic Properties
3. Materials and Methods
3.1. Materials
3.2. Methods of Characterization
3.3. Procedure for the Synthesis of Coordination Polymer 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, M.; Li, C.P.; Liu, C.S.; Fang, S.M. Design and construction of coordination polymers with mixed-ligand synthetic strategy. Coord. Chem. Rev. 2013, 257, 1282–1305. [Google Scholar] [CrossRef]
- Li, Z.Y.; Zhang, C.; Zhai, B.; Han, J.C.; Pei, M.C.; Zhang, J.J.; Zhang, F.L.; Li, S.Z.; Cao, G.X. Linking heterometallic Cu–Ln chain units with a 2-methylenesuccinate bridge to form a 2D network exhibiting a large magnetocaloric effect. CrystEngComm 2017, 19, 2702–2708. [Google Scholar] [CrossRef]
- Li, Z.Y.; Zhai, B.; Li, S.Z.; Cao, G.X.; Zhang, F.Q.; Zhang, X.F.; Zhang, F.L.; Zhang, C. Two series of lanthanide coordination polymers with 2-methylenesuccinate: Magnetic refrigerant, slow magnetic relaxation, and luminescence properties. Cryst. Growth Des. 2016, 16, 4574–4581. [Google Scholar] [CrossRef]
- Xiong, W.W.; Miao, J.W.; Ye, K.Q.; Wang, Y.; Liu, B.; Zhang, Q.C. Threading chalcogenide layers with polymer chains. Angew. Chem. Int. Ed. 2015, 54, 546–550. [Google Scholar] [CrossRef]
- Xiong, W.; Zhang, Q. Surfactants as promising media for the preparation of crystalline inorganic materials. Angew. Chem. Int. Ed. 2015, 54, 11616–11623. [Google Scholar] [CrossRef]
- Lu, H.S.; Bai, L.L.; Xiong, W.W.; Li, P.Z.; Ding, J.F.; Zhang, G.D.; Wu, T.; Zhao, Y.L.; Lee, J.M.; Yang, Y.H.; et al. Surfactant media to grow new crystalline cobalt 1,3,5-benzenetricarboxylate metal-organic frameworks. Inorg. Chem. 2014, 53, 8529–8537. [Google Scholar] [CrossRef]
- Xie, Z.; Xu, W.; Cui, X.; Wang, Y. Recent progress in metal-organic frameworks and their derived nanostructures for energy and environmental applications. ChemSusChem 2017, 10, 1645–1663. [Google Scholar] [CrossRef]
- Suh, M.P.; Park, H.J.; Prasad, T.K.; Lim, D.-W. Hydrogen storage in metal-organic frameworks. Chem. Rev. 2012, 112, 782–835. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.H.; Long, J.R. Carbon dioxide capture in metal-organic frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
- Halder, G.J.; Kepert, C.J.; Moubaraki, B.; Murray, K.S.; Cashion, J.D. Guest-dependent spin crossover in a nanoporous molecular framework material. Science 2002, 298, 1762–1765. [Google Scholar] [CrossRef]
- Li, J.R.; Sculley, J.; Zhou, H.-C. Metal–organic frameworks for separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.; Srirambalaji, R.; Kim, K. Homochiral metal–organic frameworks for asymmetric heterogeneous catalysis. Chem. Rev. 2011, 112, 1196–1231. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé, E.; Alonso, P.J.; Arauzo, A.; Luzón, J.; Bartolomé, J.; Racles, C.; Turta, C. Magnetic properties of the seven-coordinated nanoporous framework material Co(bpy)1.5(NO3)2 (bpy = 4,4′-bipyridine). Dalton Trans. 2012, 41, 10382–10389. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Campbell, M.G.; Dincă, M. Electrically conductive porous metal-organic frameworks. Angew. Chem. Int. Ed. 2016, 55, 3566–3579. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-N.; Wang, G.; Poelman, D.; Van Der Voort, P. Luminescent lanthanide MOFs: A unique platform for chemical sensing. Materials 2018, 11, 572. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, Y.; Hu, H.; Yang, Q.; Cai, J. From metal-organic frameworks to nanoporous carbons: Recent progress and prospect from energy and environmental perspectives. Nanoscale 2020, 12, 4238–4268. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Chughtai, A.H.; Ahmad, N.; Younus, H.A.; Laypkov, A.; Verpoort, F. Metal–organic frameworks: Versatile heterogeneous catalysts for efficient catalytic organic transformations. Chem. Soc. Rev. 2015, 44, 6804–6849. [Google Scholar] [CrossRef] [Green Version]
- Hoskins, B.F.; Robson, R. Infinite polymeric frameworks consisting of three dimensionally linked rod-like segments. J. Am. Chem. Soc. 1989, 111, 5962–5964. [Google Scholar] [CrossRef]
- Chakraborty, G.; Park, I.-H.; Medishetty, R.; Vittal, J.J. Two-dimensional metal-organic framework materials: Synthesis, structures, properties and applications. Chem. Rev. 2021, 121, 3751–3891. [Google Scholar] [CrossRef]
- Kostakis, G.E.; Ako, A.M.; Powell, A.K. Structural motifs and topological representation of Mn coordination clusters. Chem. Soc. Rev. 2010, 39, 2238–2271. [Google Scholar] [CrossRef] [PubMed]
- Ako, A.M.; Hewitt, I.J.; Mereacre, V.; Clérac, R.; Wernsdorfer, W.; Anson, C.E.; Powell, A.K. A ferromagnetically coupled Mn19 aggregate with a record S = 83/2 ground spin state. Angew. Chem. Int. Ed. 2006, 45, 4926–4929. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yuan, Y.; Miao, H.; Zhong, Q.; Tang, X.; Cheng, H.; Yuan, R. Structural diversity and magnetic properties of the manganese/carbazol-9-ylpropanate/N,N’-donor reaction system. Inorganica Chim. Acta 2015, 432, 64–70. [Google Scholar] [CrossRef]
- Iikura, H.; Nagata, T. Structural variation in manganase complexes: Synthesis and characterization of manganese complexes from carboxylate-containing chelating ligands. Inorg. Chem. 1998, 37, 4702–4711. [Google Scholar] [CrossRef] [PubMed]
- Albela, B.; Corbella, M.; Ribas, J.; Castro, I.; Sletten, J.; Stoeckli-Evans, H. Synthesis, structural characterization (X-ray and EXAFS), and magnetic properties of polynuclear manganese(II) complexes with chlorobenzoato bridges. Inorg. Chem. 1998, 37, 788–798. [Google Scholar] [CrossRef]
- Lin, Z.-J.; Lü, J.; Hong, M.; Cao, R. Metal–organic frameworks based on flexible ligands (FL-MOFs): Structures and applications. Chem. Soc. Rev. 2014, 43, 5867–5895. [Google Scholar] [CrossRef] [Green Version]
- Pili, S.; Rought, P.; Kolokolov, D.I.; Lin, L.; da Silva, I.; Cheng, Y.; Marsh, C.; Silverwood, I.P.; García Sakai, V.; Li, M.; et al. Enhancement of proton conductivity in non-porous metal-organic frameworks: The role of framework proton density and humidity. Chem. Mater. 2018, 30, 7593–7602. [Google Scholar] [CrossRef]
- Rought, P.; Marsh, C.; Pili, S.; Silverwood, I.P.; Garcia Sakai, V.; Li, M.; Brown, M.S.; Argent, S.P.; Vitorica-Yrezabal, I.; Whitehead, G.; et al. Modulating proton diffusion and conductivity in metal-organic frameworks by incorporation of accessible free carboxylic acid groups. Chem. Sci. 2019, 10, 1492–1499. [Google Scholar] [CrossRef] [Green Version]
- Taksand, K. Exploration of the Ionic Conduction Properties of Porous MOF Materials. Ph.D. Thesis, Université Montpellier, Montpellier, France, 2022. [Google Scholar]
- Lim, D.-W.; Kitagawa, H. Proton Transport in Metal–Organic Frameworks. Chem. Rev. 2020, 12, 8416–8467. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, D. Mesoporous carbon originated from non-permanent porous MOFs for gas storage and CO2/CH4 separation. Sci. Rep. 2014, 4, 5711. [Google Scholar] [CrossRef]
- Aiyappa, H.B.; Pachfule, P.; Banerjee, R.; Kurungot, S. Porous carbons from nonporous mofs: Influence of ligand characteristics on intrinsic properties of end carbon. Cryst. Growth Des. 2013, 13, 4195–4199. [Google Scholar] [CrossRef]
- Zaltariov, M.F.; Cazacu, M.; Sacarescu, L.; Vlad, A.; Novitchi, G.; Train, C.; Shova, S.; Arion, V.B. Oxime-bridged Mn6 clusters inserted in one-dimensional coordination polymer. Macromolecules 2016, 49, 6163–6172. [Google Scholar] [CrossRef]
- Vlad, A.; Cazacu, M.; Zaltariov, M.F.; Bargan, A.; Shova, S.; Turta, C. A 2D metal–organic framework based on dizinc coordination units bridged through both flexible and rigid ligands. J. Mol. Struct. 2014, 1060, 94–101. [Google Scholar] [CrossRef]
- Vlad, A.; Cazacu, M.; Zaltariov, M.F.; Shova, S.; Turta, C.; Airinei, A. Metallopolymeric structures containing highly flexible siloxane sequence. Polymer 2013, 54, 43–53. [Google Scholar] [CrossRef]
- Vlad, A.; Zaltariov, M.F.; Shova, S.; Novitchi, G.; Varganici, C.D.; Train, C.; Cazacu, M. Flexible linkers and dinuclear metallic nodes build up an original metal–organic framework. CrystEngComm 2013, 15, 5368–5375. [Google Scholar] [CrossRef]
- Racles, C.; Shova, S.; Cazacu, M.; Timpu, D. New highly ordered hydrophobic siloxane-based coordination polymers. Polymer 2013, 54, 6096–6104. [Google Scholar] [CrossRef]
- Hoshikawa, R.; Mitsuhashi, R.; Asato, E.; Liu, J.; Sakiyama, H. Structures of dimer-of-dimers type defect cubane tetranuclear copper(II) complexes with novel dinucleating ligands. Molecules 2022, 27, 576. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Li, D.; Li, Y.; Sakiyama, H.; Muddassir, M.; Pan, Y.; Srivastava, D.; Kumar, A. A 3,8-connected Cd(II)-based metal-organic framework as an appropriate luminescent sensor for the antibiotic sulfasalazine. CrystEngComm 2022, 24, 7157–7165. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Xue, X.; Wang, J.; Zhu, Q.; Xue, Y.; Liu, H. A two-year water-stable 2D MOF with aqueous NIR photothermal conversion ability. Dalton Trans. 2020, 50, 1374–1383. [Google Scholar] [CrossRef]
- Almenningen, A.; Bastiansen, O.; Ewing, V.; Hedberg, K.; Traetterberg, M. The molecular structure of disiloxane, (SiH3)2O. Acta Chem. Scand. 1963, 17, 2455–2460. [Google Scholar] [CrossRef]
- Owen, M.J. Why silicones behave funny. Chim. Nouv. 2005, 11, 1–11. [Google Scholar]
- Noll, W. Chemistry and Technology of Silicones; Academic Press Inc.: New York, NY, USA, 1968. [Google Scholar]
- Voronkov, M.G.; Mileshkevich, V.P.; Yuzhelevskii, Y.A. The Siloxane Bond: Physical Properties and Chemical Transformations; Springer: New York, NY, USA, 1978. [Google Scholar]
- Hillborg, H.; Tomczak, N.; Olàh, A.; Schönherr, H.; Vancso, G.J. Nanoscale hydrophobic recovery: A chemical force microscopy study of UV/ozone-treated cross-linked poly(dimethylsiloxane). Langmuir 2004, 20, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Gadzikwa, T.; Lu, G.; Stern, C.L.; Wilson, S.R.; Hupp, J.T.; Nguyen, S.B.T. Covalent surface modification of a metal–organic framework: Selective surface engineering via CuI-catalyzed Huisgen cycloaddition. Chem. Commun. 2008, 5493–5495. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.G.; Cohen, S.M. Moisture-resistant and superhydrophobic metal-organic frameworks obtained via postsynthetic modification. J. Am. Chem. Soc. 2010, 132, 4560–4561. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Zhang, Y.; Xi, J.; Zhu, Y.; Wang, N.; Xia, F.; Jiang, L. Petal effect: A Superhydrophobic state with high adhesive force. Langmuir 2008, 24, 4114–4119. [Google Scholar] [CrossRef]
- Bhushan, B.; Nosonovsky, M. The rose petal effect and the modes of superhydrophobicity. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 4713–4728. [Google Scholar] [CrossRef]
- Van der Sman, R.G.M. Predictions of glass transition temperature for hydrogen bonding biomaterials. J. Phys. Chem. B 2013, 117, 16303–16313. [Google Scholar] [CrossRef]
- Murrie, M.; Price, D.J. Molecular Magnetism. Annu. Rep. Sect. A 2007, 103, 20–38. [Google Scholar] [CrossRef]
- Cortés, R.; Drillon, M.; Solans, X.; Lezama, L. Alternating ferromagnetic-antiferromagnetic interactions in a manganese (II)-azido one-dimensional compound: [Mn(Bipy)(N3)2]. Inorg. Chem. 1997, 36, 677–683. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, J.Y.; Cheng, A.L.; Sun, Q.; Gao, E.Q.; Liu, C.M. Antiferro- and ferromagnetic interactions in Mn(II), Co(II), and Ni(II) compounds with mixed azide—carboxylate bridges. Inorg. Chem. 2009, 48, 6142–6151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Liu, C.M.; Zhang, D.Q.; Gao, S.; Zhu, D.B. Spin-canting in a 1D chain Mn(II) complex with alternating double end-on and double end-to-end azido bridging ligands. Inorg. Chem. Commun. 2007, 10, 897–901. [Google Scholar] [CrossRef]
- Zhuang, G.; Li, X.; Wen, Y.; Tian, C.; Gao, E. Structures and magnetic properties of manganese(II) compounds based on chains with simultaneous carboxylate and pseudohalide bridges. Eur. J. Inorg. Chem. 2014, 2014, 3488–3498. [Google Scholar] [CrossRef]
- Durot, S.; Policar, C.; Pelosi, G.; Bisceglie, F.; Mallah, T.; Mahy, J.P. Structural and magnetic properties of carboxylato-bridged manganese(II) complexes involving tetradentate ligands: Discrete complex and 1D polymers. Dependence of J on the nature of the carboxylato bridge. Inorg. Chem. 2003, 42, 8072–8080. [Google Scholar] [CrossRef]
- Gómez, V.; Corbella, M.; Font-Bardia, M.; Calvet, T. A Μ1,1- or Μ1,3-carboxylate bridge makes the difference in the magnetic properties of dinuclear MnII compounds. Dalton Trans. 2010, 39, 11664–11674. [Google Scholar] [CrossRef]
- Abu-Youssef, M.A.M.; Escuer, A.; Goher, M.A.S.; Mautner, F.A.; Vicente, R. Structural characterization and magnetic behaviour of the ferro-antiferromagnetic alternating manganese–azido chain [Mn(3-Et,4-Mepy)2(μ-N3)2]n (3-Et,4-Mepy = 3-Ethyl-4-Methylpyridine). Eur. J. Inorg. Chem. 1999, 1999, 687–691. [Google Scholar] [CrossRef]
- Christian, P.; Rajaraman, G.; Harrison, A.; Helliwell, M.; McDouall, J.J.W.; Raftery, J.; Winpenny, R.E.P. Synthesis and studies of a trinuclear Mn(II) carboxylate complex. Dalton Trans. 2004, 16, 2550–2555. [Google Scholar] [CrossRef]
- Rigaku, O.D. CrysAlisPro Software System, Version 1.171.38.46; Rigaku Corporation: Oxford, UK, 2015.
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Pascal, P. Recherches Magnetochimiques. Ann. Chim. Phys. 1910, 19, 5–70. [Google Scholar]










| empirical formula | C96H122Cl2Mn4N10O23Si6 |
| Fw | 2243.23 |
| space group | P21/c |
| a [Å] | 15.9092(8) |
| b [Å] | 22.2710(6) |
| c [Å] | 17.8188(9) |
| α [°] | 90 |
| β [°] | 112.866(6) |
| γ [°] | 90 |
| V [Å3] | 5817.3(5) |
| Z | 2 |
| ρcalcd [g·cm−3] | 1.281 |
| crystal size [mm] | 0.15 × 0.09 × 0.05 |
| T [K] | 293(2) |
| μ [mm−1] | 0.599 |
| 2Θ range | 3.444 to 50.054 |
| reflections collected | 35246 |
| independent reflections | 10185 [Rint = 0.0743] |
| data/restraints/parameters | 10185/161/616 |
| R1 | 0.0980 |
| wR2 | 0.2706 |
| GOF | 1.020 |
| Largest diff. peak/hole [e·Å−3] | 1.33/−0.74 |
| CCDC | 2222958 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoica, A.-C.; Damoc, M.; Shova, S.; Novitchi, G.; Dascalu, M.; Cazacu, M. A Manganese(II) 3D Metal–Organic Framework with Siloxane-Spaced Dicarboxylic Ligand: Synthesis, Structure, and Properties. Inorganics 2023, 11, 21. https://doi.org/10.3390/inorganics11010021
Stoica A-C, Damoc M, Shova S, Novitchi G, Dascalu M, Cazacu M. A Manganese(II) 3D Metal–Organic Framework with Siloxane-Spaced Dicarboxylic Ligand: Synthesis, Structure, and Properties. Inorganics. 2023; 11(1):21. https://doi.org/10.3390/inorganics11010021
Chicago/Turabian StyleStoica, Alexandru-Constantin, Madalin Damoc, Sergiu Shova, Ghenadie Novitchi, Mihaela Dascalu, and Maria Cazacu. 2023. "A Manganese(II) 3D Metal–Organic Framework with Siloxane-Spaced Dicarboxylic Ligand: Synthesis, Structure, and Properties" Inorganics 11, no. 1: 21. https://doi.org/10.3390/inorganics11010021
APA StyleStoica, A. -C., Damoc, M., Shova, S., Novitchi, G., Dascalu, M., & Cazacu, M. (2023). A Manganese(II) 3D Metal–Organic Framework with Siloxane-Spaced Dicarboxylic Ligand: Synthesis, Structure, and Properties. Inorganics, 11(1), 21. https://doi.org/10.3390/inorganics11010021