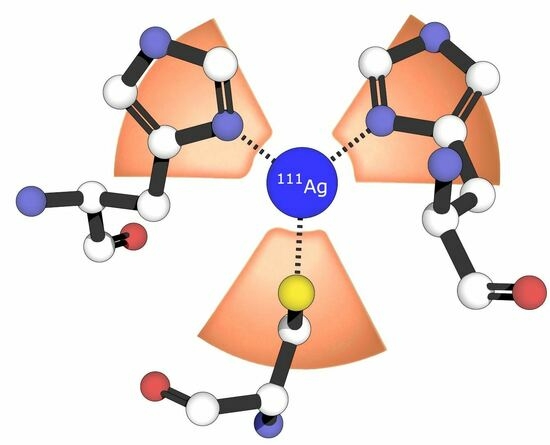
Probing the Bioinorganic Chemistry of Cu(I) with 111Ag Perturbed Angular Correlation (PAC) Spectroscopy
Abstract
:1. Introduction
2. PAC Theory
3. Examples of Applications of 111Ag PAC Spectroscopy Elucidating Cu(I) Bioinorganic Chemistry
3.1. Metal Site Structure in Small Blue Copper Proteins—Electron Transport and Transfer
3.2. Plastocynin–Photosystem I Association—Protein–Protein Interactions in Electron Transport
3.3. Metal Site Structure in Hemocyanin—Oxygen Transport
3.4. Metal Site Structure in Cu(I)-Sensing Proteins—Transcriptional Regulation
3.4.1. BxmR
3.4.2. CueR
3.5. Potential Future Applications
3.5.1. Cu(I) Binding Sites in Redox Active Proteins
3.5.2. Cu(I) Binding Sites in Cu(I) Transporting ATPases
3.5.3. Methionine Containing Cu(I) Binding Sites
3.5.4. Low Temperature Experiments
Funding
Conflicts of Interest
References
- Giedroc, D.P.; Arunkumar, A.I. Metal Sensor Proteins: Nature’s Metalloregulated Allosteric Switches. Dalton Trans. 2007, 3107–3120. [Google Scholar] [CrossRef]
- Osman, D.; Cavet, J.S. Chapter 8—Copper Homeostasis in Bacteria. In Advances in Applied Microbiology; Laskin, A.I., Sariaslani, S., Gadd, G.M., Eds.; Academic Press: Cambridge, MA, USA, 2008; Volume 65, pp. 217–247. ISBN 0065-2164. [Google Scholar]
- Lutsenko, S. Human Copper Homeostasis: A Network of Interconnected Pathways. Curr. Opin. Chem. Biol. 2010, 14, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Solomon, E.I.; Heppner, D.E.; Johnston, E.M.; Ginsbach, J.W.; Cirera, J.; Qayyum, M.; Kieber-Emmons, M.; Kjaergaard, C.H.; Hadt, R.G.; Tian, L. Copper Active Sites in Biology. Chem. Rev. 2014, 114, 3659–3853. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.A.; Lukehart, C.M. Applications of Physical Methods to Inorganic and Bioinorganic Chemistry; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Jancso, A.; Correia, J.G.; Gottberg, A.; Schell, J.; Stachura, M.; Szunyogh, D.; Pallada, S.; Lupascu, D.C.; Kowalska, M.; Hemmingsen, L. TDPAC and β-NMR Applications in Chemistry and Biochemistry. J. Phys. G Nucl. Part. Phys. 2017, 44, 064003. [Google Scholar] [CrossRef]
- Summers, M.F. 113Cd NMR Spectroscopy of Coordination Compounds and Proteins. Coord. Chem. Rev. 1988, 86, 43–134. [Google Scholar] [CrossRef]
- Bertini, I.; Luchinat, C. The Reaction Pathways of Zinc Enzymes and Related Biological Catalysts. In Bioinorganic Chemistry; University Science Books: Mill Valley, CA, USA, 1994; p. 37. [Google Scholar]
- Hay, M.T.; Milberg, R.M.; Lu, Y. Preparation and Characterization of Mercury and Silver Derivatives of an Engineered Purple Copper Center in Azurin. J. Am. Chem. Soc. 1996, 118, 11976–11977. [Google Scholar] [CrossRef]
- Santagostini, L.; Gullotti, M.; Hazzard, J.T.; Maritano, S.; Tollin, G.; Marchesini, A. Inhibition of Intramolecular Electron Transfer in Ascorbate Oxidase by Ag+: Redox State Dependent Binding. J. Inorg. Biochem. 2005, 99, 600–605. [Google Scholar] [CrossRef]
- Djoko, K.Y.; Chong, L.X.; Wedd, A.G.; Xiao, Z. Reaction Mechanisms of the Multicopper Oxidase CueO from Escherichia Coli Support Its Functional Role as a Cuprous Oxidase. J. Am. Chem. Soc. 2010, 132, 2005–2015. [Google Scholar] [CrossRef]
- Singh, S.K.; Roberts, S.A.; McDevitt, S.F.; Weichsel, A.; Wildner, G.F.; Grass, G.B.; Rensing, C.; Montfort, W.R. Crystal Structures of Multicopper Oxidase CueO Bound to Copper(I) and Silver(I): Functional Role of a Methionine-Rich Sequence. J. Biol. Chem. 2011, 286, 37849–37857. [Google Scholar] [CrossRef]
- Wilcoxen, J.; Snider, S.; Hille, R. Substitution of Silver for Copper in the Binuclear Mo/Cu Center of Carbon Monoxide Dehydrogenase from Oligotropha carboxidovorans. J. Am. Chem. Soc. 2011, 133, 12934–12936. [Google Scholar] [CrossRef]
- Chauhan, S.; Kline, C.D.; Mayfield, M.; Blackburn, N.J. Binding of Copper and Silver to Single-Site Variants of Peptidylglycine Monooxygenase Reveals the Structure and Chemistry of the Individual Metal Centers. Biochemistry 2014, 53, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Puchkova, L.V.; Broggini, M.; Polishchuk, E.V.; Ilyechova, E.Y.; Polishchuk, R.S. Silver Ions as a Tool for Understanding Different Aspects of Copper Metabolism. Nutrients 2019, 11, 1364. [Google Scholar] [CrossRef] [PubMed]
- Nardella, M.I.; Fortino, M.; Barbanente, A.; Natile, G.; Pietropaolo, A.; Arnesano, F. Multinuclear Metal-Binding Ability of the N-Terminal Region of Human Copper Transporter Ctr1: Dependence Upon pH and Metal Oxidation State. Front. Mol. Biosc. 2022, 9, 897621. [Google Scholar] [CrossRef] [PubMed]
- Kircheva, N.; Angelova, S.; Dobrev, S.; Petkova, V.; Nikolova, V.; Dudev, T. Cu+/Ag+ Competition in Type I Copper Proteins (T1Cu). Biomolecules 2023, 13, 681. [Google Scholar] [CrossRef] [PubMed]
- Hemmingsen, L.; Sas, K.N.; Danielsen, E. Biological Applications of Perturbed Angular Correlations of γ-Ray Spectroscopy. Chem. Rev. 2004, 104, 4027–4062. [Google Scholar] [CrossRef] [PubMed]
- Tröger, W. Nuclear Probes in Life Sciences. Hyperfine Interact. 1999, 120, 117–128. [Google Scholar] [CrossRef]
- Tosato, M.; Asti, M.; Di Marco, V.; Jensen, M.L.; Schell, J.; Dang, T.T.; Köster, U.; Jensen, M.; Hemmingsen, L. Towards in Vivo Applications of 111Ag Perturbed Angular Correlation of γ-Rays (PAC) Spectroscopy. Appl. Radiat. Isot. 2022, 190, 110508. [Google Scholar] [CrossRef]
- Haas, H.; Shirley, D.A. Nuclear Quadrupole Interaction Studies by Perturbed Angular Correlations. J. Chem. Phys. 1973, 58, 3339–3355. [Google Scholar] [CrossRef]
- Lerf, A.; Butz, T. Nuclear Quadrupole Interactions in Compounds Studied by Time Differential Perturbed Angular Correlations/Distributions. Hyperfine Interact. 1987, 36, 275–370. [Google Scholar] [CrossRef]
- Hansen, B.; Bukrinsky, J.T.; Hemmingsen, L.; Bjerrum, M.J.; Singh, K.; Bauer, R. Effects of the Nuclear Transformation 111Ag(I) to 111Cd(II) in a Single Crystal of Ag[111Ag](Imidazole)2NO3. Phys. Rev. B 1999, 59, 14182–14190. [Google Scholar] [CrossRef]
- Haas, H.; Röder, J.; Correia, J.G.; Schell, J.; Fenta, A.S.; Vianden, R.; Larsen, E.M.H.; Aggelund, P.A.; Fromsejer, R.; Hemmingsen, L.B.S.; et al. Free Molecule Studies by Perturbed γ- γ Angular Correlation: A New Path to Accurate Nuclear Quadrupole Moments. Phys. Rev. Lett. 2021, 126, 103001. [Google Scholar] [CrossRef] [PubMed]
- Mauk, M.R.; Gamble, R.C.; Baldeschwieler, J.D. Vesicle Targeting: Timed Release and Specificity for Leukocytes in Mice by Subcutaneous Injection. Science 1980, 207, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.; Danielsen, E.; Hemmingsen, L.; Bjerrum, M.J.; Hansson, O.; Singh, K. Interplay between Oxidation State and Coordination Geometry of Metal Ions in Azurin. J. Am. Chem. Soc. 1997, 119, 157–162. [Google Scholar] [CrossRef]
- Danielsen, E.; Kroes, S.J.; Canters, G.W.; Bauer, R.; Hemmingsen, L.; Singh, K.; Messerschmidt, A. Coordination Geometries for Monovalent and Divalent Metal Ions in [His121] Azurin. Eur. J. Biochem. 1997, 250, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, E.; Scheller, H.V.; Bauer, R.; Hemmingsen, L.; Bjerrum, M.J.; Hansson, O. Plastocyanin Binding to Photosystem I as a Function of the Charge State of the Metal Ion: Effect of Metal Site Conformation. Biochemistry 1999, 38, 11531–11540. [Google Scholar] [CrossRef] [PubMed]
- Sas, K.N.; Haldrup, A.; Hemmingsen, L.; Danielsen, E.; Øgendal, L.H. pH-Dependent Structural Change of Reduced Spinach Plastocyanin Studied by Perturbed Angular Correlation of γ-Rays and Dynamic Light Scattering. J. Biol. Inorg. Chem. 2006, 11, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Holm, J.K.; Hemmingsen, L.; Bubacco, L.; Salvato, B.; Bauer, R. Interaction and Coordination Geometries for Ag(I) in the Two Metal Sites of Hemocyanin. Eur. J. Biochem. 2000, 267, 1754–1760. [Google Scholar] [CrossRef]
- Liu, T.; Chen, X.; Ma, Z.; Shokes, J.; Hemmingsen, L.; Scott, R.A.; Giedroc, D.P. A CuI-Sensing ArsR Family Metal Sensor Protein with a Relaxed Metal Selectivity Profile. Biochemistry 2008, 47, 10564–10575. [Google Scholar] [CrossRef]
- Balogh, R.K.; Gyurcsik, B.; Jensen, M.; Thulstrup, P.W.; Köster, U.; Christensen, N.J.; Mørch, F.J.; Jensen, M.L.; Jancsó, A.; Hemmingsen, L. Flexibility of the CueR Metal Site Probed by Instantaneous Change of Element and Oxidation State from AgI to CdII. Chem. Eur. J. 2020, 26, 7451–7457. [Google Scholar] [CrossRef]
- Balogh, R.K.; Gyurcsik, B.; Jensen, M.; Thulstrup, P.W.; Köster, U.; Christensen, N.J.; Jensen, M.L.; Hunyadi-Gulyás, E.; Hemmingsen, L.; Jancsó, A. Tying Up a Loose End: On the Role of the C-Terminal CCHHRAG Fragment of the Metalloregulator CueR. ChemBioChem 2022, 23, e202200290. [Google Scholar] [CrossRef]
- Shepard, W.E.B.; Anderson, B.F.; Lewandoski, D.A.; Norris, G.E.; Baker, E.N. Copper Coordination Geometry in Azurin Undergoes Minimal Change on Reduction of Copper(II) to Copper(I). J. Am. Chem. Soc. 1990, 112, 7817–7819. [Google Scholar] [CrossRef]
- Guss, J.M.; Bartunik, H.D.; Freeman, H.C. Accuracy and Precision in Protein Structure Analysis: Restrained Least-Squares Refinement of the Structure of Poplar Plastocyanin at 1.33 Å Resolution. Acta Crystallogr. Sect. B Struct. Sci. 1992, 48, 790–811. [Google Scholar] [CrossRef]
- Shepard, W.E.B.; Kingston, R.L.; Anderson, B.F.; Baker, E.N. Structure of Apo-Azurin from Alcaligenes Denitrificans at 1.8 Å Resolution. Acta Crystallogr. Sect. D Biol. Crystallogr. 1993, 49, 331–343. [Google Scholar] [CrossRef] [PubMed]
- LLC The PyMOL Molecular Graphics System, Version 2.5.5; Schrödinger: New York, NY, USA, 2015; Available online: https://pymol.org/2/support.html? (accessed on 25 August 2023).
- Guss, J.M.; Harrowell, P.R.; Murata, M.; Norris, V.A.; Freeman, H.C. Crystal Structure Analyses of Reduced (CuI) Poplar Plastocyanin at Six pH Values. J. Mol. Biol. 1986, 192, 361–387. [Google Scholar] [CrossRef] [PubMed]
- Hazes, B.; Kalk, K.H.; Hol, W.G.J.; Magnus, K.A.; Bonaventura, C.; Bonaventura, J.; Dauter, Z. Crystal Structure of Deoxygenated Limulus Polyphemus Subunit II Hemocyanin at 2.18 Å Resolution: Clues for a Mechanism for Allosteric Regulation. Protein Sci. 1993, 2, 597–619. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.; Jensen, S.J.; Schmidt-Nielsen, B. The Angular Overlap Model Applied to the Calculation of Nuclear Quadrupole Interactions. Hyperfine Interact. 1988, 39, 203–234. [Google Scholar] [CrossRef]
- Changela, A.; Chen, K.; Xue, Y.; Holschen, J.; Outten, C.E.; O’Halloran, T.V.; Mondragón, A. Molecular Basis of Metal-Ion Selectivity and Zeptomolar Sensitivity by CueR. Science 2003, 301, 1383–1387. [Google Scholar] [CrossRef]
- Philips, S.J.; Canalizo-Hernandez, M.; Yildirim, I.; Schatz, G.C.; Mondragón, A.; O’Halloran, T.V. Allosteric Transcriptional Regulation via Changes in the Overall Topology of the Core Promoter. Science 2015, 349, 877–881. [Google Scholar] [CrossRef]
- Fromsejer, R.; Mikkelsen, K.V.; Hemmingsen, L. Dynamics of Nuclear Recoil: QM-BOMD Simulations of Model Systems Following β-Decay. Phys. Chem. Chem. Phys. 2021, 23, 25689–25698. [Google Scholar] [CrossRef]
- Gourdon, P.; Liu, X.-Y.; Skjørringe, T.; Morth, J.P.; Møller, L.B.; Pedersen, B.P.; Nissen, P. Crystal Structure of a Copper-Transporting PIB-Type ATPase. Nature 2011, 475, 59–64. [Google Scholar] [CrossRef]
- Peariso, K.; Huffman, D.L.; Penner-Hahn, J.E.; O’Halloran, T.V. The PcoC Copper Resistance Protein Coordinates Cu(I) via Novel S-Methionine Interactions. J. Am. Chem. Soc. 2003, 125, 342–343. [Google Scholar] [CrossRef]
- Wernimont, A.K.; Huffman, D.L.; Finney, L.A.; Demeler, B.; O’Halloran, T.V.; Rosenzweig, A.C. Crystal Structure and Dimerization Equilibria of PcoC, a Methionine-Rich Copper Resistance Protein from Escherichia coli. J. Biol. Inorg. Chem. 2003, 8, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Arnesano, F.; Banci, L.; Bertini, I.; Mangani, S.; Thompsett, A.R. A Redox Switch in CopC: An Intriguing Copper Trafficking Protein That Binds Copper(I) and Copper(II) at Different Sites. Proc. Natl. Acad. Sci. USA 2003, 100, 3814–3819. [Google Scholar] [CrossRef] [PubMed]
- Banci, L.; Bertini, I.; Ciofi-Baffoni, S.; Katsari, E.; Katsaros, N.; Kubicek, K.; Mangani, S. A Copper(I) Protein Possibly Involved in the Assembly of CuA Center of Bacterial Cytochrome c Oxidase. Proc. Natl. Acad. Sci. USA 2005, 102, 3994–3999. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Nadas, I.A.; Kim, M.A.; Franz, K.J. A Mets Motif Peptide Found in Copper Transport Proteins Selectively Binds Cu(I) with Methionine-Only Coordination. Inorg. Chem. 2005, 44, 9787–9794. [Google Scholar] [CrossRef]
- Zhang, L.; Koay, M.; Maher, M.J.; Xiao, Z.; Wedd, A.G. Intermolecular Transfer of Copper Ions from the CopC Protein of Pseudomonas Syringae. Crystal Structures of Fully Loaded CuICuII Forms. J. Am. Chem. Soc. 2006, 128, 5834–5850. [Google Scholar] [CrossRef]
- Bagai, I.; Liu, W.; Rensing, C.; Blackburn, N.J.; McEvoy, M.M. Substrate-Linked Conformational Change in the Periplasmic Component of a Cu(I)/Ag(I) Efflux System. J. Biol. Chem. 2007, 282, 35695–35702. [Google Scholar] [CrossRef]
- Xue, Y.; Davis, A.V.; Balakrishnan, G.; Stasser, J.P.; Staehlin, B.M.; Focia, P.; Spiro, T.G.; Penner-Hahn, J.E.; O’Halloran, T.V. Cu(I) Recognition via Cation-Pi and Methionine Interactions in CusF. Nat. Chem. Biol. 2008, 4, 107–109. [Google Scholar] [CrossRef]
- Davis, A.V.; O’Halloran, T.V. A Place for Thioether Chemistry in Cellular Copper Ion Recognition and Trafficking. Nat. Chem. Biol. 2008, 4, 148–151. [Google Scholar] [CrossRef]
- Rubino, J.T.; Riggs-Gelasco, P.; Franz, K.J. Methionine Motifs of Copper Transport Proteins Provide General and Flexible Thioether-Only Binding Sites for Cu(I) and Ag(I). J. Biol. Inorg. Chem. 2010, 15, 1033–1049. [Google Scholar] [CrossRef]
- Miranda-Blancas, R.; Avelar, M.; Rodriguez-Arteaga, A.; Sinicropi, A.; Rudiño-Piñera, E. The β-Hairpin from the Thermus thermophilus HB27 Laccase Works as a PH-Dependent Switch to Regulate Laccase Activity. J. Struct. Biol. 2021, 213, 107740. [Google Scholar] [CrossRef] [PubMed]
- Roulling, F.; Godin, A.; Feller, G. Function and Versatile Location of Met-Rich Inserts in Blue Oxidases Involved in Bacterial Copper Resistance. Biochimie 2022, 194, 118–126. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karner, V.; Jancso, A.; Hemmingsen, L. Probing the Bioinorganic Chemistry of Cu(I) with 111Ag Perturbed Angular Correlation (PAC) Spectroscopy. Inorganics 2023, 11, 375. https://doi.org/10.3390/inorganics11100375
Karner V, Jancso A, Hemmingsen L. Probing the Bioinorganic Chemistry of Cu(I) with 111Ag Perturbed Angular Correlation (PAC) Spectroscopy. Inorganics. 2023; 11(10):375. https://doi.org/10.3390/inorganics11100375
Chicago/Turabian StyleKarner, Victoria, Attila Jancso, and Lars Hemmingsen. 2023. "Probing the Bioinorganic Chemistry of Cu(I) with 111Ag Perturbed Angular Correlation (PAC) Spectroscopy" Inorganics 11, no. 10: 375. https://doi.org/10.3390/inorganics11100375
APA StyleKarner, V., Jancso, A., & Hemmingsen, L. (2023). Probing the Bioinorganic Chemistry of Cu(I) with 111Ag Perturbed Angular Correlation (PAC) Spectroscopy. Inorganics, 11(10), 375. https://doi.org/10.3390/inorganics11100375