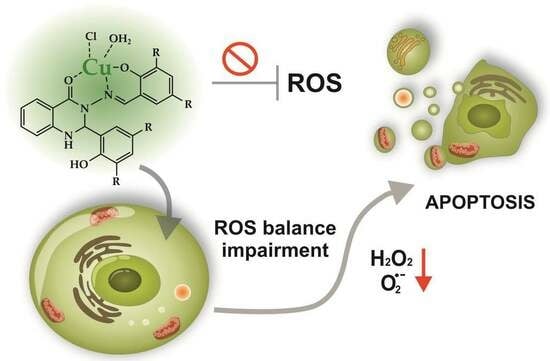
Enhancement of the Cytotoxicity of Quinazolinone Schiff Base Derivatives with Copper Coordination
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Cu(II) Complexes
2.1.1. FTIR Spectroscopy
2.1.2. UV-Vis Spectroscopy Was Proposed
2.1.3. NMR Spectroscopy
2.1.4. DFT Calculations
2.2. Determination of Antioxidant Activity
2.3. Cytotoxicity
2.4. In Vitro Antioxidant Activity
2.5. Mechanism of Cell Death
3. Materials and Methods
3.1. General Methods
3.2. Synthesis of Cu(II) Complexes (Cu-L1 and Cu-L2)
- Complex Cu-L1: Green solid; yield 64%; FT-IR (ATR, diamond): νmax 3065 (N–H), 1603 (C=O), 1575 (HC=N), 1329 (C-NO2) cm−1; UV-Vis (DMSO): λmax/nm 296, 383. Elemental analysis calculated for C21H15N5O7CuCl (%): C 46.00; H 2.76; N 12.77. Found: C 45.51; H 2.36; N 12.68; 1H NMR (600 MHz, DMSO-d6). δ: 9.48 (broad s, 1H, OH″), 8.36 (m, 2H, H2″ H4″).
- Complex Cu-L2: Brown solid; yield 65%; FT-IR (ATR, diamond): νmax 3364 (N–H), 1605 (C=O), 1582 (HC=N), cm−1; UV-Vis (DMSO): λmax/nm 298, 311, 418. Elemental analysis calculated for C23H21N3O5CuCl (%): C 53.29; H 4.08; N 8.11. Found: C 52.99; H 3.98; N 8.02; 1H NMR (600 MHz, DMSO-d6). δ: 12.74 (s, 1H, OH′), 8.14 (s, 1H, =CH), 7.25 (m, 2H, H4″, 5″).
3.3. Determination of Antioxidant Activity
3.3.1. DPPH Assay
3.3.2. FRAP Assay
3.4. Cell Culture Conditions
3.5. Cytotoxicity
3.6. Oxidative Stress Evaluation
3.7. Mechanism of Cell Death
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, B.; VanCamp, L.; Trosko, J. Platinum Compounds: A New Class of Potent Antitumour Agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lippard, S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Disc. 2005, 4, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Luo, Q.; Zhang, Y.; Jia, F.; Zhao, Y.; Wang, F. Advances in Toxicological Research of the Anticancer Drug Cisplatin. Chem. Res. Toxicol. 2019, 32, 1469–1486. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics 2022, 12, 2115–2132. [Google Scholar] [CrossRef]
- Bergamo, A.; Sava, G. Linking the future of anticancer metal-complexes to the therapy of tumour metastases. Chem. Soc. Rev. 2015, 44, 8818–8835. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Nurchi, V.M.; Lachowicz, J.I.; Crisponi, G.; Zoroddu, M.A. Noble metals in medicine: Latest advances. Coord. Chem. Rev. 2015, 284, 329–350. [Google Scholar] [CrossRef]
- Zhang, P.; Sadler, P.J. Redox-Active Metal Complexes for Anticancer Therapy. Eur. J. Inorg. Chem. 2017, 2017, 1541–1548. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. Trace elements in human physiology and pathology. Copper. Biomed. Pharmacother. 2003, 57, 386–398. [Google Scholar] [CrossRef]
- Kodama, H.; Fujisawa, C.; Bhadhprasit, W. Inherited Copper Transport Disorders: Biochemical Mechanisms, Diagnosis, and Treatment. Curr. Drug Metabol. 2012, 13, 237–250. [Google Scholar] [CrossRef]
- Mustafa, S.; Al Sharif, M. Copper (Cu) an Essential Redox-Active Transition Metal in Living System—A Review Article. Am. J. Anal. Chem. 2018, 9, 15–26. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- Thomas, C.; Mackey, M.M.; Diaz, A.A.; Cox, D.P. Hydroxyl radical is produced via the Fenton reaction in submitochondrial particles under oxidative stress: Implications for diseases associated with iron accumulation. Redox Rep. 2009, 14, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, A.; Kozlov, A. Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef]
- Tisato, F.; Marzano, C.; Porchia, M.; Pellei, M.; Santini, C. Copper in diseases and treatments, and copper-based anticancer strategies. Med. Res. Rev. 2010, 30, 708–749. [Google Scholar] [CrossRef]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in copper complexes as anticancer agents. Chem. Rev. 2013, 114, 815–862. [Google Scholar] [CrossRef]
- Akladios, F.N.; Andrew, S.D.; Parkinson, C.J. Increased generation of intracellular reactive oxygen species initiates selective cytotoxicity against the MCF-7 cell line resultant from redox active combination therapy using copper–thiosemicarbazone complexes. J. Biol. Inorg. Chem. 2016, 21, 407–419. [Google Scholar] [CrossRef]
- Rogolino, D.; Cavazzoni, A.; Gatti, A.; Tegoni, M.; Pelosi, G.; Verdolino, V.; Fumarola, C.; Cretella, D.; Petronini, P.G.; Carcelli, M. Anti-proliferative effects of copper(II) complexes with hydroxyquinoline-thiosemicarbazone ligands. Eur. J. Med. Chem. 2017, 128, 140–153. [Google Scholar] [CrossRef]
- Uddin, M.N.; Ahmed, S.S.; Rahatul Alam, S.M. Review: Biomedical applications of Schiff base metal complexes. J. Coord. Chem. 2020, 73, 3109–3149. [Google Scholar] [CrossRef]
- Chen, D.; Cui, Q.C.; Yang, H.; Dou, Q.P. Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res. 2006, 66, 10425–10433. [Google Scholar] [CrossRef]
- Guo, A.J.; Xu, X.S.; Hu, Y.H.; Wang, M.Z.; Tan, X. Effects of ternary complexes of copper with salicylaldehyde-aminoacid Schiff base coordination compounds on the proliferation of BGC823 cells. Chin. J. Cancer 2010, 29, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Szymański, P.; Frączek, T.; Markowicz, M.; Mikiciuk-Olasik, E. Development of copper based drugs, radiopharmaceuticals and medical materials. BioMetals 2012, 25, 1089–1112. [Google Scholar] [CrossRef] [PubMed]
- Iakovidis, I.; Delimaris, I.; Piperakis, S.M. Copper and Its Complexes in Medicine: A Biochemical Approach. Mol. Biol. Int. 2011, 2011, 594529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, H.; Yan, M.; Wang, H.; Zhang, C. Novel copper complexes as potential proteasome inhibitors for cancer treatment. Mol. Med. Rep. 2016, 15, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Shagufta; Ahmad, I. Transition metal complexes as proteasome inhibitors for cancer treatment. Inorg. Chim. Acta 2020, 506, 119521. [Google Scholar] [CrossRef]
- Ashok, U.P.; Kollur, S.P.; Arun, B.P.; Sanjay, C.; Suresh, K.S.; Anil, N.; Baburao, H.V.; Markad, D.; Castro, J.O.; Frau, J.; et al. In vitro anticancer activity of 4(3H)-quinazolinone derived Schiff base and its Cu(II), Zn(II) and Cd(II) complexes: Preparation, X-ray structural, spectral characterization and theoretical investigations. Inorg. Chim. Acta 2020, 511, 119846. [Google Scholar] [CrossRef]
- Li, S.X.; Luo, P.; Jiang, Y.M. Copper complexes with 4(3H)-quinazolinone: Thermal gravimetric analysis and anticancer activity of [Cu(L)2(H2O)2(NO3)2], [Cu(L–)(NO3)]n, and [Cu(L)2(H2O)2(Cl)2]. Russ. J. Coord. Chem. 2017, 43, 238–243. [Google Scholar] [CrossRef]
- Lazou, M.; Tarushi, A.; Gritzapis, P.; Psomas, G. Transition metal complexes with a novel guanine-based (E)-2-(2-(pyridin-2-ylmethylene)hydrazinyl)quinazolin-4(3H)-one: Synthesis, characterization, interaction with DNA and albumins and antioxidant activity. J. Inorg. Biochem. 2020, 206, 111019. [Google Scholar] [CrossRef]
- Ubale, P.; Mokale, S.; More, S.; Waghamare, S.; More, V.; Munirathinam, N.; Dilipkumar, S.; Das, R.K.; Reja, S.; Helavi, V.B.; et al. Evaluation of in vitro anticancer, antimicrobial and antioxidant activities of new Cu(II) complexes derived from 4(3H)-quinazolinone: Synthesis, crystal structure and molecular docking studies. J. Mol. Struct. 2022, 1251, 131984. [Google Scholar] [CrossRef]
- Brissos, R.F.; Caubet, A.; Gamez, P. Possible DNA-Interacting Pathways for Metal-Based Compounds Exemplified with Copper Coordination Compounds. Eur. J. Inorg. Chem. 2015, 2015, 2633–2645. [Google Scholar] [CrossRef]
- Hricovíniová, Z.; Hricovíni, M.; Kozics, K. New series of quinazolinone derived Schiff’s bases: Synthesis, spectroscopic properties and evaluation of their antioxidant and cytotoxic activity. Chem. Pap. 2018, 72, 1041–1053. [Google Scholar] [CrossRef]
- Hricovíniová, J.; Hricovíniová, Z.; Kozics, K. Antioxidant, Cytotoxic, Genotoxic, and DNA-Protective Potential of 2,3-Substituted Quinazolinones: Structure-Activity Relationship Study. Int. J. Mol. Sci. 2021, 22, 610. [Google Scholar] [CrossRef] [PubMed]
- Zahedifard, M.; Faraj, F.L.; Paydar, M.; Looi, C.Y.; Hajrezaei, M.; Hasanpourghadi, M.; Kamalidehghan, B.; Majid, N.A.; Ali, H.M.; Abdulla, M.A. Synthesis, characterization and apoptotic activity of quinazolinone Schiff base derivatives toward MCF-7 cells via intrinsic and extrinsic apoptosis pathways. Sci. Rep. 2015, 5, 11544. [Google Scholar] [CrossRef] [PubMed]
- Gudasi, K.B.; Patil, S.A.; Vadavi, R.S.; Shenoy, R.V.; Nethaji, M. Crystal structure of 2-[2-hydroxy-3-methoxyphenyl]-3-[2-hydroxy-3-methoxybenzylamino]-1,2-dihydroquinazolin-4(3H)-one and the synthesis, spectral and thermal investigation of its transition metal complexes. Trans. Metal Chem. 2006, 31, 586–592. [Google Scholar] [CrossRef]
- Tapabashi, N.O.; Taha, N.I.; El-Subeyhi, M. Design, Microwave Assisted Synthesis of Some Schiff Bases Derivatives of Congo Red and Conventional Preparation of Their Structurally Reversed Analogous Compounds. Int. J. Org. Chem. 2021, 11, 35–45. [Google Scholar] [CrossRef]
- Nielsen, I.B.; Petersen, M.Å.; Lammich, L.; Nielsen, M.B.; Andersen, L.H. Absorption Studies of Neutral Retinal Schiff Base Chromo-phores. J. Phys. Chem. A 2006, 110, 12592–12596. [Google Scholar] [CrossRef]
- Crompton, E.M.; Lewis, F.D. Positional effects of the hydroxy substituent on the photochemical and photophysical behavior of 3- and 4-hydroxystilbene. Photochem. Photobiol. Sci. 2004, 3, 660–668. [Google Scholar] [CrossRef]
- Salga, M.; Sada, M.; Mustapha, I.A. Influence of Steric Hindrance on The Antioxidant Activity of Some Schiff Base Ligands And Their Copper(II) Complexes. Orient. J. Chem. 2014, 30, 1529–1534. [Google Scholar] [CrossRef]
- Turan, N.; Adigüzel, R.; Buldurun, K.; Bursal, E. Spectroscopic, Thermal and Antioxidant Properties of Novel Mixed Ligand-Metal Complexes Obtained from Saccharinate Complexes and Azo Dye Ligand (mnppa). Int. J. Pharmacol. 2016, 12, 92–100. [Google Scholar] [CrossRef]
- Buldurun, K.; Turan, N.; Aras, A.; Mantarci, A.; Turkan, F.; Bursal, E. Spectroscopic and Structural Characterization, Enzyme Inhibitions, and Antioxidant Effects of New Ru(II) and Ni(II) Complexes of Schiff Base. Chem. Biodivers. 2019, 16, e1900243. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Nunes, I.J.; Ferreira, W.V.; Tomasini, P.P.; Trindade, C.; Martins, C.C.; Wilhelm, E.A.; Oliboni, R.d.S.; Netz, P.A.; Stieler, R.; et al. Antioxidant and Anticancer Potential of the New Cu(II) Complexes Bearing Imine-Phenolate Ligands with Pendant Amine N-Donor Groups. Pharmaceuticals 2023, 15, 376. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.J.; Irwin, W.; Diaz, E.D.; Howard-Cofield, E.E.; Krejsa, C.M.; Slaughter, M.R.; Gao, E.B.; Kaludercic, N.; Angeline, E.A.; Bernardi, E.P.; et al. High concordance of drug-induced human hepatotoxicity with in vitro cytotoxicity measured in a novel cell-based model using high content screening. Arch. Toxicol. 2006, 80, 580–604. [Google Scholar] [CrossRef] [PubMed]
- Mazuryk, O.; Suzenet, F.; Kieda, C.; Brindell, M. Biological effect of nitroimidazole derivative of polypyridyl ruthenium complex on cancer and endothelial cells. Metallomics 2015, 7, 553–566. [Google Scholar] [CrossRef]
- Gurgul, I.; Janczy-Cempa, E.; Mazuryk, O.; Lekka, M.; Łomzik, M.; Suzenet, F.; Gros, P.G.; Brindell, M. Inhibition of Metastasis by Polypyridyl Ru(II) Complexes through Modification of Cancer Cell Adhesion–In Vitro Functional and Molecular Studies. J. Med. Chem. 2022, 65, 10459–10470. [Google Scholar] [CrossRef]
- Kesavan, M.P.; Vinoth Kumar, G.G.; Dhaveethu Raja, J.; Anitha, K.; Karthikeyan, S.; Rajesh, J. DNA interaction, antimicrobial, antioxidant and anticancer studies on Cu(II) complexes of Luotonin A. J. Photochem. Photobiol. B Biol. 2017, 167, 20–28. [Google Scholar] [CrossRef]
- Peña, Q.; Lorenzo, J.; Sciortino, G.; Rodríguez-Calado, S.; Maréchal, J.-D.; Bayóna, P.; Simaan, A.J.; Iranzo, O.; Capdevila, M.; Palaciosa, Ò. Studying the reactivity of “old” Cu(II) complexes for “novel” anticancer purposes. J. Inorg. Biochem. 2019, 195, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Sawant, A.V.; Prassanawar, S.S.; Panda, D. Copper-Plumbagin Complex Produces Potent Anticancer Effects by Depolymerizing Microtubules and Inducing Reactive Oxygen Species and DNA Damage. ACS Omega 2023, 8, 3221–3235. [Google Scholar] [CrossRef]
- Sîrbu, A.; Palamarciuc, O.; Babak, M.V.; Lim, J.M.; Ohui, K.; Enyedy, E.A.; Shova, S.; Darvasiová, D.; Rapta, P.; Ang, W.H.; et al. Copper(II) thiosemicarbazone complexes induce marked ROS accumulation and promote nrf2-mediated antioxidant response in highly resistant breast cancer cells. Dalton Trans. 2017, 46, 3833–3847. [Google Scholar] [CrossRef]
- Schumacker, P.T. Reactive oxygen species in cancer: A dance with the devil. Cancer Cell 2015, 27, 156–157. [Google Scholar] [CrossRef]
- Kang, K.A.; Piao, M.J.; Ryu, Y.S.; Hyun, Y.J.; Park, J.E.; Shilnikova, K.; Zhen, A.X.; Kang, H.K.; Koh, Y.S.; Jeong, Y.J.; et al. Luteolin induces apoptotic cell death via antioxidant activity in human colon cancer cells. Int. J. Oncol. 2017, 51, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Rev. B.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Austin, A.; Petersson, G.A.; Frisch, M.J.; Dobek, F.J.; Scalmani, G.; Throssell, K. A density functional with spherical atom dispersion terms. J. Chem. Theor. Comput. 2012, 8, 4989–5007. [Google Scholar] [CrossRef] [PubMed]
- Dunning, T.H.; Hay, P.J. Gaussian Basis Sets for Molecular Calculations. In Methods of Electronic Structure Theory, Modern Theoretical Chemistry; Schaefer, H.F., Ed.; Springer: Boston, MA, USA, 1977; Volume 3, pp. 1–28. [Google Scholar]
- Locatelli, M.; Gindro, R.; Travaglia, F.; Coïsson, J.D.; Rinaldi, M.; Arlorio, M. Development of a free software for the correct interpretation of data. Food Chem. 2009, 114, 889–897. [Google Scholar] [CrossRef]
- Gupta, D. Methods for determination of antioxidant capacity: A review. Int. J. Pharm. Sci. Res. 2015, 6, 546–566. [Google Scholar] [CrossRef]
- Gurgul, I.; Mazuryk, O.; Łomzik, M.; Gros, P.C.; Rutkowska-Zbik, D.; Brindell, M. Unexplored features of Ru(II) polypyridyl complexes-towards combined cytotoxic and antimetastatic activity. Metallomics 2020, 12, 784–793. [Google Scholar] [CrossRef]






| Compound | IC50/μM | |||
|---|---|---|---|---|
| A549 | MCF-7 | |||
| MTT | Resazurin | MTT | Resazurin | |
| Cu-L1 | 2.4 ± 0.2 | 4.2 ± 0.4 | 1.35 ± 0.04 | 2.1 ± 0.2 |
| Cu-L2 | 1.2 ± 0.3 | 2.2 ± 0.3 | 0.56 ± 0.08 | 1.0 ± 0.2 |
| L1 | >32 | >32 | >32 | >32 |
| L2 | >32 | >32 | >32 | >32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurgul, I.; Hricovíniová, J.; Mazuryk, O.; Hricovíniová, Z.; Brindell, M. Enhancement of the Cytotoxicity of Quinazolinone Schiff Base Derivatives with Copper Coordination. Inorganics 2023, 11, 391. https://doi.org/10.3390/inorganics11100391
Gurgul I, Hricovíniová J, Mazuryk O, Hricovíniová Z, Brindell M. Enhancement of the Cytotoxicity of Quinazolinone Schiff Base Derivatives with Copper Coordination. Inorganics. 2023; 11(10):391. https://doi.org/10.3390/inorganics11100391
Chicago/Turabian StyleGurgul, Ilona, Jana Hricovíniová, Olga Mazuryk, Zuzana Hricovíniová, and Małgorzata Brindell. 2023. "Enhancement of the Cytotoxicity of Quinazolinone Schiff Base Derivatives with Copper Coordination" Inorganics 11, no. 10: 391. https://doi.org/10.3390/inorganics11100391
APA StyleGurgul, I., Hricovíniová, J., Mazuryk, O., Hricovíniová, Z., & Brindell, M. (2023). Enhancement of the Cytotoxicity of Quinazolinone Schiff Base Derivatives with Copper Coordination. Inorganics, 11(10), 391. https://doi.org/10.3390/inorganics11100391