Exploring the Structure and Properties of VwSeyTe2−y Mixed Crystals in the VTe2–VSe2 System †
Abstract
:1. Introduction
2. Results and Discussion
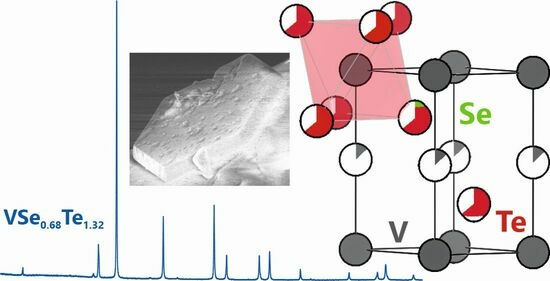
2.1. Crystal Structure Description of VwSeyTe2−y
2.2. Elemental Analysis by Means of Energy-Dispersive X-ray Diffraction
2.3. Powder X-ray Diffraction Analysis of Compounds in the V–Se–Te System
2.4. Magnetic Measurements of VSe0.72Te1.28
2.5. Thermal Analyses of VSe0.72Te1.28
3. Materials and Methods
3.1. Syntheses
3.2. Single-Crystal X-ray Diffraction Experiments and Structure Resolution
3.3. Powder X-ray Diffraction Experiments and Refinement
3.4. Scanning Electron Microscopy (SEM) with Energy-Dispersive X-ray Spectroscopy Measurements (EDX)
3.5. Measurements of the Magnetic Properties
3.6. Thermal Analyses of VSe0.72Te1.28
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dunn, J.P.; Stenger, H.G.; Wachs, I.E. Oxidation of SO2 over Supported Metal Oxide Catalysts. J. Catal. 1999, 181, 233–243. [Google Scholar] [CrossRef]
- Sanap, P.P.; Gupta, S.P.; Kahandal, S.S.; Gunjakar, J.L.; Lokhande, C.D.; Sankapal, B.R.; Said, Z.; Bulakhe, R.N.; Man Kim, J.; Bhalerao, A.B. Exploring vanadium-chalcogenides toward solar cell application: A review. J. Ind. Eng. Chem. 2023, 129, 124–142. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Z. Carbon-coated vanadium selenide as anode for lithium-ion batteries and sodium-ion batteries with enhanced electrochemical performance. Mater. Lett. 2017, 189, 152–155. [Google Scholar] [CrossRef]
- Hoschek, E.; Klemm, W. Vanadinselenide. Z. Anorg. Allg. Chem. 1939, 242, 49–62. [Google Scholar] [CrossRef]
- Ehrlich, P. Über die binären Systeme des Titans mit den Elementen Stickstoff, Kohlenstoff, Bor und Beryllium. Z. Anorg. Chem. 1949, 259, 1–41. [Google Scholar] [CrossRef]
- Biltz, W.; Köcher, A. Beiträge zur systematischen Verwandtschaftslehre. 88. Über das System Vanadium/Schwefel. Z. Anorg. Allg. Chem. 1939, 241, 324–337. [Google Scholar] [CrossRef]
- Wiegers, G.A. Physical properties of first-row transition metal dichalcogenides and their intercalates. Phys. B C 1980, 99, 151–165. [Google Scholar] [CrossRef]
- Nakahira, M.; Hayashi, K. Characterization of the layered transition-metal dichalcogenides with octahedral coordination. Mat. Res. Bull. 1978, 13, 1403–1408. [Google Scholar] [CrossRef]
- Bronsema, K.D.; Bus, G.W.; Wiegers, G.A. The crystal structure of vanadium ditelluride, V1+xTe2. J. Solid State Chem. 1984, 53, 415–421. [Google Scholar] [CrossRef]
- van Bruggen, C.F.; Haas, C. Magnetic susceptibility and electrical properties of VSe2 single crystals. Solid State Commun. 1976, 20, 251–254. [Google Scholar] [CrossRef]
- DiSalvo, F.J.; Waszczak, J.V. Magnetic studies of VSe2. Phys. Rev. B 1981, 23, 457–461. [Google Scholar] [CrossRef]
- Won, D.; Kiem, D.H.; Cho, H.; Kim, D.; Kim, Y.; Jeong, M.Y.; Seo, C.; Kim, J.; Park, J.-G.; Han, M.J.; et al. Polymorphic Spin, Charge, and Lattice Waves in Vanadium Ditelluride. Adv. Mater. 2020, 32, 1906578. [Google Scholar] [CrossRef]
- Duvjir, G.; Jung, J.-A.; Ly, T.T.; Lam, N.H.; Chang, Y.J.; Lee, S.; Kim, H.; Kim, J. Fine structure of the charge density wave in bulk VTe2. APL Mater. 2022, 10, 111102. [Google Scholar] [CrossRef]
- Huang, Z.-L.; Bensch, W.; Benea, D.; Ebert, H. Anion substitution effects on structure and magnetism in the chromium chalcogenide Cr5Te8—Part I: Cluster glass behavior in trigonal Cr(1+x)Q2 with basic cell (Q = Te, Se; Te:Se = 7:1). J. Solid State Chem. 2004, 177, 3245–3253. [Google Scholar] [CrossRef]
- Lotgering, F.K.; Gorter, E.W. Solid solutions between ferromagnetic and antiferromagnetic compounds with NiAs structure. J. Phys. Chem. Solids 1957, 3, 238–249. [Google Scholar] [CrossRef]
- Tsubokawa, I. The Magnetic Properties of Chromium-Tellurium-Selenium System. J. Phys. Soc. Jpn. 1956, 11, 662–665. [Google Scholar] [CrossRef]
- Wontcheu, J.; Bensch, W.; Mankovsky, S.; Polesya, S.; Ebert, H.; Kremer, R.K.; Brücher, E. Anion substitution effects on the structure and magnetism of the chromium chalcogenide Cr5Te8—Part III: Structures and magnetism of the high-temperature modification Cr(1+x)Q2 and the low-temperature modification Cr(5+x)Q8 (Q = Te, Se; Te:Se = 5:3). J. Solid State Chem. 2008, 181, 1492–1505. [Google Scholar] [CrossRef]
- Arnaud, Y.; Chevreton, M. Etude comparative des composés TiX2 (X = S, Se, Te). Structures de TiTe2 et TiSeTe. J. Solid State Chem. 1981, 39, 230–239. [Google Scholar] [CrossRef]
- Rimmington, H.P.B.; Balchin, A.A. The growth by iodine vapour transport techniques and the crystal structures of layer compounds in the series TiSxSe2−x, TiSxTe2−x, TiSexTe2−x. J. Cryst. Growth 1974, 21, 171–181. [Google Scholar] [CrossRef]
- Dey, S.; Lee, J.; Britto, S.; Stratford, J.M.; Keyzer, E.N.; Dunstan, M.T.; Cibin, G.; Cassidy, S.J.; Elgaml, M.; Grey, C.P. Exploring Cation–Anion Redox Processes in One-Dimensional Linear Chain Vanadium Tetrasulfide Rechargeable Magnesium Ion Cathodes. J. Am. Chem. Soc. 2020, 142, 19588–19601. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.M.; Scott, J.H.J.; Joy, D.C. Scanning Electron Microscopy and X-ray Microanalysis, 4th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B: Condens. Matter. 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent developments of the program FULLPROF. Comm. Powder Diffr. (IUCr) Newsl. 2001, 26, 12. [Google Scholar]
- Vegard, L. Die Konstitution der Mischkristalle und die Raumfüllung der Atome. Z. Phys. 1921, 5, 17–26. [Google Scholar] [CrossRef]
- Goldschmidt, V.M. Die Gesetze der Krystallochemie. Naturwissenschaften 1926, 14, 477–485. [Google Scholar] [CrossRef]
- Shannon, R. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Lueken, H. Magnetochemie; Teubner Verlag: Stuttgart, Germany, 1999. [Google Scholar]
- Sakuma, T.; Xianglian; Siagian, S.; Basar, K.; Takahashi, H.; Igawa, N.; Kamishima, O. Correlation effects among thermal displacements of atoms in VSe by diffuse neutron scattering measurement. J. Therm. Anal. Calorim. 2010, 99, 173–176. [Google Scholar] [CrossRef]
- Sharygin, V.V.; Ripp, G.S.; Yakovlev, G.A.; Seryotkin, Y.V.; Karmanov, N.S.; Izbrodin, I.A.; Grokhovsky, V.I.; Khromova, E.A. Uakitite, VN, a New Mononitride Mineral from Uakit Iron Meteorite (IIAB). Minerals 2020, 10, 150. [Google Scholar] [CrossRef]
- Lengauer, W.; Ettmayer, P. Lattice parameters and thermal expansion of δ-VN1−x from 298–1000 K. Monatsh. Chem. 1986, 117, 713–719. [Google Scholar] [CrossRef]
- Ettmayer, P.; Schebesta, W.; Vendl, A.; Kieffer, R. Beitrag zur Kenntnis des Systems Vanadin—Chrom—Stickstoff. Monatsh. Chem. 1978, 109, 929–941. [Google Scholar] [CrossRef]
- APEX2, v2014.11-0; Bruker AXS: Madison, WI, USA, 2015.
- Farrugia, L. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- WinXPOW Powder Diffraction Software, 3.7.0.0; STOE & Cie GmbH: Darmstadt, Germany, 2021.








| Formula | V1.13Se0.72Te1.28 | V1.10Se0.42Te1.58 |
|---|---|---|
| form wt. (g mol−1) | 277.74 | 290.81 |
| space group | m1 | m1 |
| a (Å) | 3.626(2) | 3.633(7) |
| c (Å) | 6.290(2) | 6.365(12) |
| volume (Å3) | 71.6(1) | 72.7(3) |
| Z | 1 | 1 |
| calc. density (g cm−3) | 6.441 | 6.639 |
| µ (mm−1) | 25.541 | 24.140 |
| F(000) | 117 | 122 |
| θ range (°) | 3.24–30.40 | 3.20–31.00 |
| index range | −5 ≤ h ≤ 4 −5 ≤ k ≤ 5 −8 ≤ l ≤ 8 | −5 ≤ h ≤ 4 −4 ≤ k ≤ 5 −9 ≤ l ≤ 7 |
| reflections collected | 1049 | 585 |
| independent reflections | 108 | 111 |
| refinement method | Full matrix least squares on F2 | |
| data/restraints/parameters | 108/0/11 | 111/0/14 |
| goodness-of-fit on F2 | 1.208 | 0.987 |
| final R indices [I > 2σ(I)] | R1 = 0.021; wR2 = 0.049 | R1 = 0.020; wR2 = 0.042 |
| R indices (all data) | R1 = 0.023; wR2 = 0.050 | R1 = 0.021; wR2 = 0.042 |
| Rint | 0.025 | 0.029 |
| largest diff. peak and hole (e−Å−3) | 1.454 and −1.198 | 0.807 and −0.904 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurig, S.; Ketter, F.; Frommelius, A.; Hakala, B.V.; van Leusen, J.; Friese, K.; Dronskowski, R. Exploring the Structure and Properties of VwSeyTe2−y Mixed Crystals in the VTe2–VSe2 System. Inorganics 2023, 11, 481. https://doi.org/10.3390/inorganics11120481
Kurig S, Ketter F, Frommelius A, Hakala BV, van Leusen J, Friese K, Dronskowski R. Exploring the Structure and Properties of VwSeyTe2−y Mixed Crystals in the VTe2–VSe2 System. Inorganics. 2023; 11(12):481. https://doi.org/10.3390/inorganics11120481
Chicago/Turabian StyleKurig, Sophia, Fabian Ketter, Anne Frommelius, B. Viliam Hakala, Jan van Leusen, Karen Friese, and Richard Dronskowski. 2023. "Exploring the Structure and Properties of VwSeyTe2−y Mixed Crystals in the VTe2–VSe2 System" Inorganics 11, no. 12: 481. https://doi.org/10.3390/inorganics11120481
APA StyleKurig, S., Ketter, F., Frommelius, A., Hakala, B. V., van Leusen, J., Friese, K., & Dronskowski, R. (2023). Exploring the Structure and Properties of VwSeyTe2−y Mixed Crystals in the VTe2–VSe2 System. Inorganics, 11(12), 481. https://doi.org/10.3390/inorganics11120481