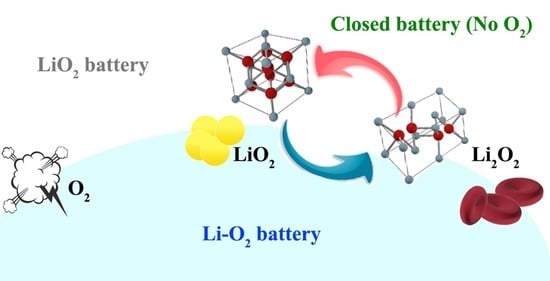
Reversible Conversion between Lithium Superoxide and Lithium Peroxide: A Closed “Lithium–Oxygen” Battery
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of a Pd-rGO Hybrid Catalyst
3.2. Structure Characterization of a Pd-rGO Catalyst
3.3. Battery Fabrication and Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Jean-Marie, T. Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 2011, 11, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Geng, D.; Ding, N.; Hor, T.A.; Chien, S.W.; Liu, Z.; Wuu, D.; Sun, X.; Zong, Y. From Lithium-Oxygen to Lithium-Air Batteries: Challenges and Opportunities. Adv. Energy Mater. 2016, 6, 1502164. [Google Scholar] [CrossRef]
- Grande, L.; Paillard, E.; Hassoun, J.; Park, J.-B.; Lee, Y.-J.; Sun, Y.-K.; Passerini, S.; Scrosati, B. The lithium/air battery: Still an emerging system or a practical reality? Adv. Mater. 2015, 27, 784–800. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, Y.; Tang, C.; Wang, J.; Zhang, Q.; Wang, Y.; Zhang, J. Atomic Modulation and Structure Design of Carbons for Bifunctional Electrocatalysis in Metal–Air Batteries. Adv. Mater. 2019, 31, 1803800. [Google Scholar] [CrossRef]
- Léon, A.; Fiedler, A.; Blum, M.; Benkert, A.; Meyer, F.; Yang, W.; Bär, M.; Scheiba, F.; Ehrenberg, H.; Weinhardt, L.; et al. Valence Electronic Structure of Li2O2, Li2O, Li2CO3, and LiOH Probed by Soft X-ray Emission Spectroscopy. J. Phys. Chem. C 2017, 121, 5460–5466. [Google Scholar] [CrossRef]
- Cui, Q.; Zhang, Y.; Ma, S.; Peng, Z. Li2 O2 oxidation: The charging reaction in the aprotic Li-O2 batteries. Sci. Bull. 2015, 60, 1227–1234. [Google Scholar] [CrossRef]
- Zhang, X.; Gong, Y.; Li, S.; Sun, C. Porous Perovskite La0.6Sr0.4Co0.8Mn0.2O3 Nanofibers Loaded with RuO2 Nanosheets as an Efficient and Durable Bifunctional Catalyst for Rechargeable Li–O2 Batteries. ACS Catal. 2017, 7, 7737–7747. [Google Scholar] [CrossRef]
- Ma, R.; Sasaki, T. Nanosheets of oxides and hydroxides: Ultimate 2D charge-bearing functional crystallites. Adv. Mater. 2010, 22, 5082–5104. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, C.; Li, Z.; Xie, Y. Vacancy engineering for tuning electron and phonon structures of two-dimensional materials. Adv. Energy Mater. 2016, 6, 1600436. [Google Scholar] [CrossRef]
- Lim, H.-D.; Lee, B.; Zheng, Y.; Hong, J.; Kim, J.; Gwon, H.; Ko, Y.; Lee, M.; Cho, K.; Kang, K. Rational design of redox mediators for advanced Li–O2 batteries. Nat. Energy 2016, 1, 16066. Available online: https://www.nature.com/articles/nenergy201666#supplementary-information (accessed on 23 December 2015). [CrossRef]
- Cheng, F.; Zhang, T.; Zhang, Y.; Du, J.; Han, X.; Chen, J. Enhancing electrocatalytic oxygen reduction on MnO(2) with vacancies. Angew. Chem. Int. Ed. Engl. 2013, 52, 2474–2477. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Li, F.; Ma, C.; Wang, Y.; Ren, Y.; Yang, W.; Ma, Z.; Li, J.; Chen, Y.; Kim, Y.; et al. Graphene–Co3O4 nanocomposite as an efficient bifunctional catalyst for lithium–air batteries. J. Mater. Chem. A 2014, 2, 7188–7196. [Google Scholar] [CrossRef]
- Cheng, F.; Chen, J. Metal-air batteries: From oxygen reduction electrochemistry to cathode catalysts. Chem. Soc. Rev. 2012, 41, 2172–2192. [Google Scholar] [CrossRef] [PubMed]
- Thotiyl, M.M.O.; Freunberger, S.A.; Peng, Z.; Bruce, P.G. The carbon electrode in nonaqueous Li-O2 cells. J. Am. Chem. Soc. 2013, 135, 494–500. [Google Scholar] [CrossRef]
- McCloskey, B.D.; Speidel, A.; Scheffler, R.; Miller, D.C.; Viswanathan, V.; Hummelshøj, J.S.; Nørskov, J.K.; Luntz, A.C. Twin Problems of Interfacial Carbonate Formation in Nonaqueous Li-O2 Batteries. J. Phys. Chem. Lett. 2012, 3, 997–1001. [Google Scholar] [CrossRef]
- Peng, Z.; Freunberger, S.A.; Chen, Y.; Bruce, P.G. A reversible and higher-rate Li-O2 battery. Science 2012, 337, 563–566. [Google Scholar] [CrossRef]
- Asadi, M.; Sayahpour, B.; Abbasi, P.; Ngo, A.T.; Karis, K.; Jokisaari, J.R.; Liu, C.; Narayanan, B.; Gerard, M.; Yasaei, P.; et al. A lithium–oxygen battery with a long cycle life in an air-like atmosphere. Nature 2018, 555, 502–506. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Gasteiger, H.A.; Crumlin, E.; McGuire, R., Jr.; Shao-Horn, Y. Electrocatalytic Activity Studies of Select Metal Surfaces and Implications in Li-Air Batteries. J. Electrochem. Soc. 2010, 157, A1016–A1025. [Google Scholar] [CrossRef]
- Black, R.; Oh, S.H.; Lee, J.-H.; Yim, T.; Adams, B.; Nazar, L.F. Screening for superoxide reactivity in Li-O2 batteries: Effect on Li2O2/LiOH crystallization. J. Am. Chem. Soc. 2012, 134, 2902–2905. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Wang, J.; McKee, W.C.; Xu, Y.; Peng, Z. Potential-Dependent Generation of O2– and LiO2 and Their Critical Roles in O2 Reduction to Li2O2 in Aprotic Li–O2 Batteries. J. Phys. Chem. C 2016, 120, 3690–3698. [Google Scholar] [CrossRef]
- Peng, Z.; Chen, Y.; Bruce, P.G.; Xu, Y. Direct Detection of the Superoxide Anion as a Stable Intermediate in the Electroreduction of Oxygen in a Non-Aqueous Electrolyte Containing Phenol as a Proton Source. Angew. Chem. Int. Ed. Engl. 2015, 54, 8165–8168. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Black, R.; Pomerantseva, E.; Lee, J.-H.; Nazar, L. Synthesis of a metallic mesoporous pyrochlore as a catalyst for lithium-O2 batteries. Nat. Chem. 2012, 4, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Yuan, Y.; Tan, G.; Liu, C.; Cheng, M.; Yurkiv, V.; Bi, X.; Long, F.; Friedrich, C.R.; Mashayek, F.; et al. Tuning Li2O2 Formation Routes by Facet Engineering of MnO2 Cathode Catalysts. J. Am. Chem. Soc. 2019, 141, 12832–12838. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Ding, W.; Li, Z.; Su, R.; Zhang, X.; Wang, J.; Zhou, J.; Wang, Z.; Gao, Y.; Li, S.; et al. Inverse Spinel Cobalt–Iron Oxide and N-Doped Graphene Composite as an Efficient and Durable Bifuctional Catalyst for Li–O2 Batteries. ACS Catal. 2018, 8, 4082–4090. [Google Scholar] [CrossRef]
- Lim, H.-D.; Lee, B.; Bae, Y.; Park, H.; Ko, Y.; Kim, H.; Kim, J.; Kang, K. Reaction chemistry in rechargeable Li-O2 batteries. Chem. Soc. Rev. 2017, 46, 2873–2888. [Google Scholar] [CrossRef]
- Gao, R.; Liang, X.; Yin, P.; Wang, J.; Lee, Y.L.; Hu, Z.; Liu, X. An amorphous LiO2-based Li-O2 battery with low overpotential and high rate capability. Nano Energy 2017, 41, 535–542. [Google Scholar] [CrossRef]
- Lu, J.; Lee, Y.J.; Luo, X.; Lau, K.C.; Asadi, M.; Wang, H.-H.; Brombosz, S.; Wen, J.; Zhai, D.; Chen, Z.; et al. A lithium-oxygen battery based on lithium superoxide. Nature 2016, 529, 377–382. [Google Scholar] [CrossRef]
- Gao, X.; Chen, Y.; Johnson, L.R.; Jovanov, Z.P.; Bruce, P.G. A rechargeable lithium–oxygen battery with dual mediators stabilizing the carbon cathode. Nat. Energy 2017, 2, 17118. [Google Scholar] [CrossRef]
- Li, F.; Tang, D.-M.; Chen, Y.; Golberg, D.; Kitaura, H.; Zhang, T.; Yamada, A.; Zhou, H. Ru/ITO: A carbon-free cathode for nonaqueous Li-O2 battery. Nano Lett. 2013, 13, 4702–4707. [Google Scholar] [CrossRef]
- Liu, T.; Leskes, M.; Yu, W.; Moore, A.J.; Zhou, L.; Bayley, P.M.; Kim, G.; Grey, C.P. Cycling Li-O2 batteries via LiOH formation and decomposition. Science 2015, 350, 530–533. [Google Scholar] [CrossRef] [Green Version]
- Khetan, A.; Luntz, A.; Viswanathan, V. Trade-Offs in Capacity and Rechargeability in Nonaqueous Li-O2 Batteries: Solution-Driven Growth versus Nucleophilic Stability. J. Phys. Chem. Lett. 2015, 6, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Tang, J.; Li, F.; Liu, X.; Yamauchi, Y.; Ishida, M.; Zhou, H. A Synergistic System for Lithium-Oxygen Batteries in Humid Atmosphere Integrating a Composite Cathode and a Hydrophobic Ionic Liquid-Based Electrolyte. Adv. Funct. Mater. 2016, 26, 3291–3298. [Google Scholar] [CrossRef]
- Lutz, L.; Yin, W.; Grimaud, A.; Alves Dalla Corte, D.; Tang, M.; Johnson, L.; Azaceta, E.; Sarou-Kanian, V.; Naylor, A.J.; Hamad, S.; et al. Capacity Na–O2 Batteries: Key Parameters for Solution-Mediated Discharge. J. Phys. Chem. C 2016, 120, 20068–20076. [Google Scholar] [CrossRef]
- Gittleson, F.S.; Jones, R.E.; Ward, D.K.; Foster, M.E. Oxygen solubility and transport in Li–air battery electrolytes: Establishing criteria and strategies for electrolyte design. Energ. Environ. Sci. 2017, 10, 1167–1179. [Google Scholar] [CrossRef]
- Qiao, Y.; Jiang, K.; Deng, H.; Zhou, H. A high-energy-density and long-life lithium-ion battery via reversible oxide–peroxide conversion. Nat. Catal. 2019, 2, 1035–1044. [Google Scholar] [CrossRef]
- Han, X.-B.; Kannari, K.; Ye, S. In situ surface-enhanced Raman spectroscopy in Li–O2 battery research. Curr. Opin. Electrochem. 2019, 17, 174–183. [Google Scholar] [CrossRef]
- Lau, K.C.; Qiu, D.; Luo, X.; Greeley, J.; Curtiss, L.A.; Lu, J.; Amine, K. Theoretical Exploration of Various Lithium Peroxide Crystal Structures in a Li-Air Battery. Energies 2015, 8, 529–548. [Google Scholar] [CrossRef]
- Johnson, L.; Li, C.; Liu, Z.; Chen, Y.; Freunberger, S.; Ashok, P.C.; Praveen, B.B.; Dholakia, K.; Tarascon, J.-M.; Bruce, P.G. The role of LiO2 solubility in O2 reduction in aprotic solvents and its consequences for Li-O2 batteries. Nat. Chem. 2014, 6, 1091–1099. [Google Scholar] [CrossRef]
- Qiao, Y.; He, Y.; Wu, S.; Jiang, K.; Li, X.; Guo, S.; He, P.; Zhou, H. MOF-Based Separator in an Li–O2 Battery: An Effective Strategy to Restrain the Shuttling of Dual Redox Mediators. ACS Energy Lett. 2018, 3, 463–468. [Google Scholar] [CrossRef]
- Peng, Z.; Freunberger, S.; Hardwick, L.; Chen, Y.; Giordani, V.; Bardé, F.; Novák, P.; Graham, D.; Tarascon, J.-M.; Bruce, P.G. Oxygen reactions in a non-aqueous Li+ electrolyte. Angew. Chem. Int. Ed. Engl. 2011, 50, 6351–6355. [Google Scholar] [CrossRef]






| Process | Theoretical Specific Capacity (mAh g−1) | |
|---|---|---|
| O2 +Li+ + e−→LiO2 | Pre-Discharge | 688.4021 |
| O2 + 2Li+ + e−→Li2O2 | Open-Discharge (With O2) | 1168.5377 |
| LiO2→Li2O2 | Closed-Cycle (No O2) | 480.1356 |
| M (mol∙g−1) | N (mol) | C (mAh g−1) | 1/C (ng nAh−1) | |
|---|---|---|---|---|
| LiO2 | 38.9388 | 1 | 688.4021 | 1.4526 |
| Li2O2 | 45.8788 | 2 | 1168.5377 | 0.8558 |
| 2LiOH | 47.8946 | 4 | 2238.7083 | 0.4467 |
| LiO2→Li2O2 | 6.9400 | 1 | 480.1356 | 2.0827 |
| Pd-rGO in Ar | 2.0522 | |||
| rGO in O2 | 0.3427 |
| Sample | ΔQ (e−) | ΔO2 (nmol) | n (e− mol−1) |
|---|---|---|---|
| Closed Pre | 7.46 × 10−6 | 7053.46 (O2→LiO2) | 1.06 |
| Closed Discharge | 0.76 × 10−6 | 762.06 (O2→LiO2) | 1.00 |
| Closed Charge | 0.71 × 10−6 | 711.06 (LiO2→O2) | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Gao, R.; Liu, X. Reversible Conversion between Lithium Superoxide and Lithium Peroxide: A Closed “Lithium–Oxygen” Battery. Inorganics 2023, 11, 69. https://doi.org/10.3390/inorganics11020069
Wang J, Gao R, Liu X. Reversible Conversion between Lithium Superoxide and Lithium Peroxide: A Closed “Lithium–Oxygen” Battery. Inorganics. 2023; 11(2):69. https://doi.org/10.3390/inorganics11020069
Chicago/Turabian StyleWang, Junkai, Rui Gao, and Xiangfeng Liu. 2023. "Reversible Conversion between Lithium Superoxide and Lithium Peroxide: A Closed “Lithium–Oxygen” Battery" Inorganics 11, no. 2: 69. https://doi.org/10.3390/inorganics11020069
APA StyleWang, J., Gao, R., & Liu, X. (2023). Reversible Conversion between Lithium Superoxide and Lithium Peroxide: A Closed “Lithium–Oxygen” Battery. Inorganics, 11(2), 69. https://doi.org/10.3390/inorganics11020069