Inorganic Sorbents for Wastewater Treatment from Radioactive Contaminants
Abstract
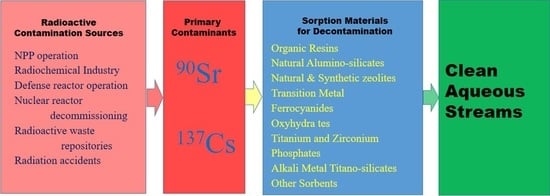
:1. Introduction
2. Results and Discussion
3. Materials and Methods
- Purolite C 100—a gel-type strongly acidic sulfonic cation exchanger. Grain size was 0.315–1.25 mm, manufactured by the “Purolite” Company, UK;
- Bent-Ru is bentonite clay, Belgorod region, Russia;
- Bent-Gr is bentonite clay, Milos Island, Greece;
- Cl-UKR is clinoptilolite of the Sokirnitsa deposit, Ukraine;
- Cl-RUS is clinoptilolite of the Kholinsky deposit, Chita Region, Russia.
- NaA is sodium form of the type A zeolite, TU 2163-003-15285215-2006, manufactured by Ishimbay Specialized Chemical Plant of Catalysts (ISCPC), Bashkiria, Russia;
- NaX is sodium form of the type X zeolite, TU 2163-077-05766575-99, manufactured by Ishimbay Specialized Chemical Plant of Catalysts (ISCPC), Bashkiria, Russia;
- Termoksid-3K is spheric-shaped granulated zirconium oxyhydrate, manufactured by the JSC “Termoksid,” Russia;
- MDM is manganese (III, IV) oxide-based granulated sorbent, TU 2641-001-51255813-2007, manufactured by the Frumkin Institute of Physical Chemistry and Electrochemistry of the Russian Academy of Sciences (IPCE RAS), Moscow, Russia;
- Termoksid-3A is spheric-shaped granulated zirconium phosphate, manufactured by the JSC “Termoksid,” Russia;
- Termoksid-35 is spheric-shaped granulated nickel-potassium ferrocyanide on a zirconium oxyhydrate carrier, the ferrocyanide phase content is 28–32 wt.%, manufactured by the JSC “Termoksid,” Russia
- FNS is granulated nickel-potassium ferrocyanide on a silica gel carrier, the ferrocyanide phase content is 8–10 wt.%, manufactured by the IPCE RAS, Moscow, Russia;
- FND is finely dispersed nickel-potassium ferrocyanide on a natural aluminosilicate carrier, the ferrocyanide phase content is 15–20 wt.%, manufactured by the IPCE RAS, Moscow, Russia;
- SRM-Sr is granular sorption-reagent barium silicate-based material, granule size of 0.25—3.0 mm, the specific surface area of 326 m2 g−1; manufactured at the Institute of Chemistry, Far Eastern Branch of the Russian Academy of Sciences, Vladivostok, Russia;
- BAU is granulated activated birch wood-based carbon. Granule size of 1.2–3.6 mm, 1.2–3.6 mm, bulk densityof 0.12 g cm−3, the specific surface area of 700–800 m2 g−1; micropores’ volume of 0.22–0.25 cm3 g−1, mesopores’ volume of 0.08–0.10 cm3 g−1, macropores’ volume of 1.35–1.45 cm3 g−1,, manufactured by the NPO “Sorbent,” Russia;
- NWC is a granulated activated coconut shell-based carbon. Granule size of 0.42–1.7 mm, bulk density of 0.18–0.52 g cm−3, a specific surface area of 350–400 m2 g−1; the micropores’ volume of 0.33–0.35 cm3 g−1, mesopores’ volume of 0.03–0.05 cm3 g−1, macropores’ volume of less than 0.01 cm3 g−1, the adsorption capacity of 240 mg g1, produced by NWCarbon (India).
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Looking Back to go Forward. In Proceedings of the an International Conference on Chernobyl, STI/PUB/1312. Vienna, Austria, 6–7 September 2005; ISBN 978–92–0–110807–4.
- The Fukushima Daiichi Accident; Report by the Director General; STI/PUB/1710; IAEA: Vienna, Austria, 2015; ISBN 978–92–0–107015–9.
- Abbasi, W.A.; Streat, M. Adsorption of uranium from aqueous solutions using activated carbon. Separ. Sci. Technol. 1994, 29, 1217–1230. [Google Scholar] [CrossRef]
- Bulut, Y.; Tez, Z. Removal of heavy metals from aqueous solution by sawdust adsorption. J. Environ. Sci. 2007, 19, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Kutahyali, C.; Eral, M. Selective adsorption of uranium from aqueous solutions using activated carbon prepared from charcoal by chemical activation. Sep. Purif. Technol. 2004, 40, 109–114. [Google Scholar] [CrossRef]
- Olorundare, O.F.; Krause, R.W.M.; Okonkwo, J.O.; Mamba, B.B. Potential application of activated carbon from maize tassel for the removal of heavy metals in water. J. Phys. Chem. Earth 2012, 50–52, 104–110. [Google Scholar] [CrossRef]
- Someda, H.H.; Ezz El-Din, M.R.; Sheha, R.R.; El Naggar, H.A. Application of a carbonized apricot stone for the treatment of some radioactive nuclei. J. Radioanal. Nucl. Chem. 2002, 254, 373–378. [Google Scholar] [CrossRef]
- Borai, E.H.; Hilal, M.A.; Attallah, M.F.; Shehata, F.A. Improvement of radioactive liquid waste treatment efficiency by sequential cationic and anionic ion exchangers. Radiochim. Acta. 2008, 96, 441–447. [Google Scholar] [CrossRef]
- Application of Ion Exchange Processes for the Treatment of Radioacative Waste and Management of Spent Ion Exchangers; Technical Reports Series, No. 408; IAEA: Vienna, Austria, 2002; p. 202.
- Ferrah, N.; Abderrahim, O.; Didi, M.A. Comparative study of Cd2+ ions sorption by both Lewatit TP 214 and Lewatit TP 208 resins: Kinetic, equilibrium and thermodynamic modelling. Chem. J. 2015, 5, 6–13. [Google Scholar]
- Kadous, A.; Didi, M.A.; Villemin, D. Removal of uranium (VI) from acetate medium using Lewatit TP 260 resin. J. Radioanal. Nucl. Chem. 2020, 288, 553–561. [Google Scholar] [CrossRef]
- Venkatesan, K.A.; Shyaqemla, K.V.; Antony, M.P.; Srinivasan, T.G.; Vasudeva, P.R. Batch and dynamic extraction of uranium from nitric acid medium by commercial phosphonic acid resin Tulsion CH-96. J. Radioanal. Nucl. Chem. 2008, 275, 563–570. [Google Scholar] [CrossRef]
- Cheira, M.F.; Atia, B.M.; Kouraim, M.N. Uranium(VI) recovery from acidic leach liquor by Ambersep 920U SO4 resin: Kinetic, equilibrium and thermodynamic studies. J. Radiat. Res. Appl. Sci. 2017, 10, 307–319. [Google Scholar] [CrossRef] [Green Version]
- Kosari, M.; Sepehrian, H.; Ahangari, R. Uranium Removal from Aqueous Solution Using Ion-exchange Resin DOWEX 2 × 8 in the Presence of Sulfate Anions. Int. J. Eng. 2016, 29, 1677–1683. [Google Scholar] [CrossRef]
- Hartmann, E.; Geckeis, H.; Rabung, T.; Lützenkirchen, J.; Fanghänel, T. Sorption of radionuclides onto natural clay rocks. Radiochim. Acta. 2008, 96, 699–707. [Google Scholar] [CrossRef]
- Lujaniene, G.; Šapolaite, J.; Radžiute, E.; Ščiglo, T.; Beneš, P.; Štamberg, K.; Vopalka, D. Effect of natural clay components on sorption of Cs, Pu and Am by the clay. J. Radioanal. Nucl. Chem. 2010, 286, 353–359. [Google Scholar] [CrossRef]
- Milyutin, V.V.; Gelis, V.M.; Nekrasova, N.A.; Kononenko, O.A.; Vezentsev, A.I.; Volovicheva, N.A.; Korol’kova, S.V. Sorption of Cs, Sr, U and Pu radionuclides on natural and modified clays. Radiochemistry 2012, 54, 75–78. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Kovalchuk, I.; Buszewski, B. The separation of uranium ions by natural and modified diatomite from aqueous solution. J. Hazard. Mater. 2010, 181, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Borai, E.H.; Harjula, R.; Paajanen, A. Efficient removal of cesium from low-level radioactive liquid waste using natural and impregnated zeolite minerals. J. Hazard. Mater. 2009, 172, 416–422. [Google Scholar] [CrossRef]
- Misaelides, P. Application of natural zeolites in environmental remediation: A short review. Microporous Mesoporous Mater. 2001, 144, 15–18. [Google Scholar] [CrossRef]
- Osmanloglu, A.E. Treatment of radioactive liquid waste for sorption on natural zeolite in Turkey. J. Hazard. Mater. 2009, 137, 332–335. [Google Scholar] [CrossRef]
- Kwon, S.; Choi, Y.; Sigh, B.K. Selective and rapid capture of Sr2+ with LTA zeolites: Effect of crystal sizes and mesoporosity. Appl. Surf. Sci. 2020, 506, 15. [Google Scholar] [CrossRef]
- Sigh, B.K.; Tomar, R.; Kumar, S.; Jain, A.; Tomar, B.S.; Manchanda, V.K. Sorption of 137Cs, 133Ba and 154Eu by synthesized sodium aluminosilicate (Na-AS). J. Hazard. Mater. 2010, 178, 771–776. [Google Scholar] [CrossRef]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A Review. J. Hazard. Mater. 2012, 211–212, 317–331. [Google Scholar] [CrossRef]
- Kuznetsova, V.A.; Generalova, V.A. The study of sorption properties of iron, manganese, titanium, aluminum and silicone oxides with respect to 90Sr and 137Cs. Radiochemistry 2000, 42, 154–157. [Google Scholar]
- Valsala, T.P.; Joseph, A.; Sonar, N.L.; Sonavane, M.S.; Shah, J.G.; Raj, K.A.; Venugopal, V. Separation of strontium from low level radioactive waste solutions using hydrous manganese dioxide composite materials. J. Nucl. Mater. 2010, 404, 138–143. [Google Scholar] [CrossRef]
- Singh, B.K.; Tomar, R.; Kumar, S.; Kar, A.S.; Tomar, B.S.; Ramanathan, S.; Manchanda, V.K. Role of the humic acid for sorption of radionuclides by synthesized titania. Radiochim. Acta 2014, 102, 255–261. [Google Scholar] [CrossRef]
- Ivanets, A.; Milyutin, V.; Shashkova, I.; Kitikova, N.; Nekrasova, N.; Radkevich, A. Sorption of stable and radioactive Cs(I), Sr(II), Co(II) ions on Ti-Ca-Mg phosphates. J. Radioanal. Nucl. Chem. 2020, 324, 1115–1123. [Google Scholar] [CrossRef]
- Thakkar, R.; Chudasama, U. Synthesis and characterization of zirconium titanium phosphate and its application in separation of metal ions. J. Hazard. Mater. 2009, 172, 129–137. [Google Scholar] [CrossRef]
- Decaillon, J.G.; Andres, Y.; Mokili, B.M.; Abbe, J.C.; Tournoux, M.; Patarin, J. Study of the ion exchange selectivity of layered titanosilicate Na3(Na,H)Ti2O2[Si2O6]2×2H2O, AM-4, for strontium. Solvent Extr. Ion Exch. 2002, 20, 273–291. [Google Scholar] [CrossRef]
- Hobbs, D.T.; Barnes, M.J.; Pulmano, R.L.; Marshall, K.M.; Edwards, T.B.; Bronikowski, M.G.; Fink, S.D. Strontium and Actinide Separations from High Level Nuclear Waste Solutions Using Monosodium Titanate. 1. Simulant Testing. Sep. Sci. Technol. 2005, 40, 3093–3111. [Google Scholar] [CrossRef] [Green Version]
- Lehto, J.; Brodkin, L.; Harjula, R.; Tusa, E. Separation of radioactive strontium from alkaline nuclear waste solutions with the highly effective ion exchanger SrTreat. Nucl. Technol. 1999, 127, 81–87. [Google Scholar] [CrossRef]
- Milyutin, V.V.; Nekrasova, N.A.; Yanicheva, N.Y.; Kalashnikova, G.O.; Ganicheva, Y.Y. Sorption of cesium and strontium radionuclides onto crystalline alkali metal titanosilicates. Radiochemistry 2017, 59, 65–69. [Google Scholar] [CrossRef]
- Park, Y.; Shin, W.S.; Reddy, G.S.; Shin, S.J.; Choi, S.J. Use of nanocrystalline silicotitanate for the removal of Cs, Co and Sr from low-level liquid radioactive waste. J. Nanoelectron. Optoelectron. 2010, 5, 238–242. [Google Scholar] [CrossRef]
- Solbra, N.; Allison, S.; Waite, S.; Mihalovsky, A.; Bortun, L.; Clearfield, A. Cesium and strontium ion exchange on the framework titanium silicate M2Ti2O3SiO4×nH2O (M = H, Na). Environ. Sci. Technol. 2001, 35, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.D.; Li, F.Z.; Zhao, X.; Yun, G.C. Preparing high-loaded potassium cobalt hexacyanoferrate/silica composite for radioactive wastewater treatment. Nucl. Technol. 2009, 165, 200–208. [Google Scholar] [CrossRef]
- Milonjic, I.; Bispo, M.; Fedoroff, C.V. Sorption of cesium on cooper hexacyanoferrate/polymer/silica composites in batch and dynamic conditions. J. Radioanal. Nucl. Chem. 2002, 252, 497–501. [Google Scholar] [CrossRef]
- Milyutin, V.V.; Kononenko, O.A.; Mikheev, S.V.; Gelis, V.M. Sorption of cesium on finely dispersed composite ferrocyanide sorbents. Radiochemistry 2010, 52, 281–283. [Google Scholar] [CrossRef]
- Chaptman, D.M.; Roe, A.L. Synthesis, characterization and crystal chemistry of microporous titanium-silicate materials. Zeolites 1990, 10, 730–737. [Google Scholar] [CrossRef]
- Duff, M.C.; Hanter, D.B.; Hobbs, D.T.; Fink, S.D.; Dai, Z.; Bradey, J.P. Mechanism of strontium and uranium removal from high-level radioactive waste simulant solution by the sorbent monosodium titanate. Environ. Sci. Technol. 2007, 38, 5201–5207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Park, S.M.; Jeon, E.K.; Baek, K. Selective and irreversible adsorption mechanism of cesium on illite. Appl. Geochem. 2017, 85, 188–193. [Google Scholar] [CrossRef]
- Leontieva, G.V. Structural modification of manganese (III, IV) oxides in synthesis of sorbents selective for strontium. Russ. J. Appl. Chem. 1997, 70, 1615–1618. [Google Scholar]
- Alby, D.; Charnay, C.; Heran, M.; Prelot, B.; Zajac, J. Recent developments in nanostructured inorganic materials for sorption of cesium and strontium: Synthesis and shaping, sorption capacity, mechanisms, and selectivity—A review. J. Hazard. Mater. 2018, 344, 511–530. [Google Scholar] [CrossRef]
- Massoni, N.; Koch, R.J.; Hertz, A.; Campayo, L.; Moloney, M.P.; Misture, S.T.; Grandjean, A. Structural effects of calcination on Cs-exchanged copper hexacyanoferrate (Cs,K)2CuFe(CN)6 loaded on mesoporous silica particles. J. Nucl. Mater. 2020, 528, 151887. [Google Scholar] [CrossRef]
- Li, Y.L.; Zeidman, B.D.; Hu, S.Y.; Henager, C.H., Jr.; Besmann, T.M.; Grandjean, A. A physics-based mesoscale phase-field model for predicting the uptake kinetics of radionuclides in hierarchical nuclear wasteform materials. Comput. Mater. Sci. 2019, 159, 103–109. [Google Scholar] [CrossRef]
- Abdel-Rahman, R.O.; Ibrahim, H.A.; Hanafy, M.; Abdel-Monem, N.M. Assessment of synthetic zeolite NaA-X as sorbing barrier for strontium in a radioactive disposal facility. Chem. Eng. J. 2010, 157, 100–112. [Google Scholar] [CrossRef]
- Hassan, N.M.; Adu-Wusu, K.; Marra, J.C. Resorcinol—Formaldehyde adsorption of cesium from Hanford waste solutions. J. Radioanal. Nucl. Chem. 2005, 262, 579–586. [Google Scholar] [CrossRef]
- Sergienko, V.I.; Avramenko, V.A.; Glushchenko, V.Y. Sorption Technology of LRW Treatment. J. Ecotechnol. Res. 1995, 1, 152. [Google Scholar]
- Andryushchenko, N.D.; Safonov, A.V.; Babich, T.L.; Ivanov, P.V.; Konevnik, Y.V.; Kondrashova, A.A.; Proshin, I.M.; Zakharova, E.V. Sorption characteristics of materials of the filtration barrier in upper aquifers contaminated with radionuclides. Radiochemistry 2017, 9, 414–424. [Google Scholar] [CrossRef]
- Fuks, L.; Herdzik-Koniecko, I. Vermiculite as a potential component of the engineered barriers in low- and medium-level radioactive waste repositories. Appl. Clay Sci. 2018, 161, 139–150. [Google Scholar] [CrossRef]
- Osipov, V.I.; Sokolov, V.N. Clays and their properties. Composition, structure, and formation of properties. Moscow GEOS RF 2013. [Google Scholar]
- Olphen, V. Data Handbook for Clay Materials and other Non-Metallic Minerals; Pergamon Press: Oxford, UK, 1979; Volume 28. [Google Scholar]
- Cornell, R.M. Adsorption of cesium on minerals: A review. J. Radioanal. Nucl. Chem. 1993, 171, 483–500. [Google Scholar] [CrossRef]
- Maskalchuk, L.N.; Milyutin, V.V.; Nekrasova, N.A.; Leontieva, T.G.; Baklay, A.A.; Belousov, P.E.; Krupskaya, V.V. Aluminosilicate Sorbents Based on Clay-Salt Slimes from JSC «Belaruskali» for Sorption of Cesium and Strontium Radionuclides. Radiochemistry 2020, 62, 381–386. [Google Scholar] [CrossRef]
- Tananaev, I.V.; Seifer, G.B.; Kharitonov, Y.Y. The chemistry of ferrocyanides. Moscow Nauka RF 1971. [Google Scholar]
- Marhol, M. Ion Exchangers in Analytical Chemistry; Elsevier Scientific Pub. Co.: New York, NY, USA, 1982. [Google Scholar]
- Sharygin, L.M. Thermal Resistant Inorganic Sorbents; UrO RAS: Ekaterinburg, Russia, 2012. [Google Scholar]
- Milyutin, V.V.; Gelis, V.M.; Leonov, N.B. Kinetic sorption studies of cesium and strontium radionuclides by the sorbents of different classes. Radiochemistry 1998, 40, 418–420. [Google Scholar]
- Feng, Q.; Kanoh, H.; Ooi, K. Manganese oxide porous crystals. J. Mater. Chem. 1999, 9, 319–333. [Google Scholar] [CrossRef]
- Avramenko, V.A.; Zheleznov, V.V.; Kaplun, E.V.; Sokol’nitskaya, T.A.; Yukhkam, A.A. Sorption Recovery of Strontium from Seawater. Radiochemistry 2001, 43, 433–436. [Google Scholar] [CrossRef]
- Avramenko, V.A.; Sergienko, V.I.; Sokol’nitskaya, T.A. Application of sorption-reagent materials in the technology of liquid radioactive waste treatment. Theor. Found. Chem. Eng. 2016, 50, 593–597. [Google Scholar] [CrossRef]








| Sorbent Type | Brand Name, Designation | Kd 137Cs, cm3 g−1 in 0.1 mol dm−3 NaNO3 | Kd 90Sr, cm3 g−1 in 0.01 mol dm−3 CaCl2 |
|---|---|---|---|
| Sulfonic cation exchanger | Purolite C 100 | 450 | 290 |
| Bentonite clay | Bent–Ru | 9100 | 40 |
| Bentonite clay | Bent-Gr | 2300 | 35 |
| Clinoptilolite | Cl-UKR | 1600 | 70 |
| Clinoptilolite | Cl-RUS | 1800 | 65 |
| Zeolite of type A | NaA | 8900 | 350 |
| Zeolite of type X | NaX | 1800 | 320 |
| Zirconium(IV) oxyhydrate | Termoksid-3K | 150 | 210 |
| Manganese (III, IV) oxyhydrate | MDM | 290 | 5600 |
| Zirconium phosphate | Termoksid-3A | 1800 | 440 |
| Nickel-potassium ferrocyanide | Termoksid-35 | 1.2 × 105 | 35 |
| Nickel-potassium ferrocyanide | FNS | 8.4 × 104 | 10 |
| Nickel-potassium ferrocyanide | FND | 8.1 × 104 | 25 |
| Barium silicate | SRM-Sr | <10 | 185 |
| Activated charcoal | BAU | 440 | <5 |
| Activated charcoal | NWC | 470 | <5 |
| No. | Characteristics | Unit of Measurement | Value of the Characteristic | |
|---|---|---|---|---|
| FNS | Termoksid-35 | |||
| 1 | Type of the adsorption isotherm according to IUPAC | - | IV | IV |
| 2 | Specific surface area, by BET | m2 g−1 | 167 ± 2 | 328 ± 4 |
| 3 | Specific volume of pores below 300 nm in diameter | cm3 g−1 | 0.60 ± 0.01 | 0.21 ± 0.01 |
| 4 | Specific volume of micropores | cm3 g−1 | 0.04 ± 0.01 | 0.08 ± 0.01 |
| 5 | Prevailing pore diameter | nm | 23 ± 1 | 2.8 ± 0.3 |
| Sorbent Name | Bent–Ru | Cl-UKR | Purolite C 100 | NaA | MDM | FNS |
|---|---|---|---|---|---|---|
| Kd 137Cs, cm3 g−1 | 2.5 × 104 | 1.8 × 104 | 1650 | 8500 | - | 2.4 × 104 |
| Kd 90Sr, cm3 g−1 | 330 | 580 | 1880 | 4400 | 8600 | - |
| Sorbent Name | Bent–Ru | Cl-UKR | NaA | MDM | FNS | SRM-Sr |
|---|---|---|---|---|---|---|
| Kd 137Cs, cm3 g−1 | 3500 | 1700 | 280 | ˂10 | 1.1 × 104 | ˂10 |
| Kd 90Sr, cm3 g−1 | ˂10 | ˂10 | ˂10 | 590 | ˂10 | 4.0 × 104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nekrasova, N.A.; Milyutin, V.V.; Kaptakov, V.O.; Kozlitin, E.A. Inorganic Sorbents for Wastewater Treatment from Radioactive Contaminants. Inorganics 2023, 11, 126. https://doi.org/10.3390/inorganics11030126
Nekrasova NA, Milyutin VV, Kaptakov VO, Kozlitin EA. Inorganic Sorbents for Wastewater Treatment from Radioactive Contaminants. Inorganics. 2023; 11(3):126. https://doi.org/10.3390/inorganics11030126
Chicago/Turabian StyleNekrasova, Natalya A., Vitaly V. Milyutin, Victor O. Kaptakov, and Evgeny A. Kozlitin. 2023. "Inorganic Sorbents for Wastewater Treatment from Radioactive Contaminants" Inorganics 11, no. 3: 126. https://doi.org/10.3390/inorganics11030126
APA StyleNekrasova, N. A., Milyutin, V. V., Kaptakov, V. O., & Kozlitin, E. A. (2023). Inorganic Sorbents for Wastewater Treatment from Radioactive Contaminants. Inorganics, 11(3), 126. https://doi.org/10.3390/inorganics11030126