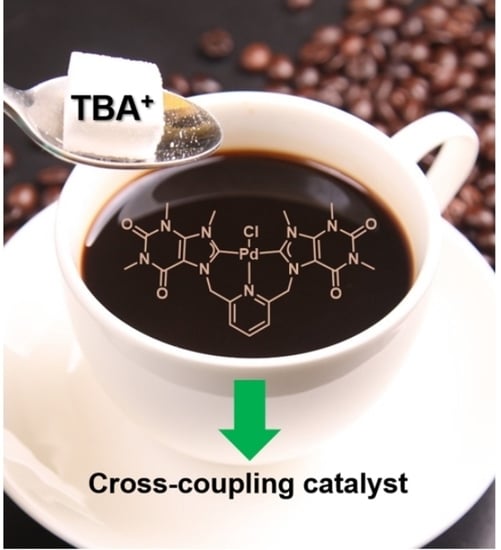
Catalysis of a Bis-Caffeine Palladium(II) NHC-Pincer Complex
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arduengo, A.J.; Harlow, R.L.; Kline, M. A stable crystalline carbene. J. Am. Chem. Soc. 1991, 113, 361–363. [Google Scholar] [CrossRef]
- Hillier, A.C.; Sommer, W.J.; Yong, B.S.; Petersen, J.L.; Cavallo, L.; Nolan, S.P. A Combined Experimental and Theoretical Study Examining the Binding of N-Heterocyclic Carbenes (NHC) to the Cp*RuCl (Cp* = η5-C5Me5) Moiety: Insight into Stereoelectronic Differences between Unsaturated and Saturated NHC Ligands. Organometallics 2003, 22, 4322–4326. [Google Scholar] [CrossRef]
- Huynh, H.V.; Han, Y.; Jothibasu, R.; Yang, J.A. 13C NMR Spectroscopic Determination of Ligand Donor Strengths Using N-Heterocyclic Carbene Complexes of Palladium(II). Organometallics 2009, 28, 5395–5404. [Google Scholar] [CrossRef]
- Huynh, H.V.; Lam, T.T.; Luong, H.T.T. Anion influences on reactivity and NMR spectroscopic features of NHC precursors. RSC Adv. 2018, 8, 34960–34966. [Google Scholar] [CrossRef] [PubMed]
- Mazars, F.; Zaragoza, G.; Delaude, L. Caffeine and theophylline as sustainable, biosourced NHC ligand precursors for efficient palladium-catalyzed Suzuki–Miyaura cross-coupling reactions. J. Organomet. Chem. 2022, 978, 122489. [Google Scholar] [CrossRef]
- Marquard, A.N.; Slaymaker, L.E.; Hamers, R.J.; Goldsmith, R.H. Investigation of activity, stability, and degradation mechanism of surface-supported Pd-PEPPSI complexes for Suzuki-Miyaura coupling. Mol. Catal. 2017, 429, 10–17. [Google Scholar] [CrossRef]
- Organ, M.G.; Chass, G.A.; Fang, D.-C.; Hopkinson, A.C.; Valente, C. Pd-NHC (PEPPSI) Complexes: Synthetic Utility and Computational Studies into Their Reactivity. Synthesis 2008, 2008, 2776–2797. [Google Scholar] [CrossRef]
- Rahman, M.M.; Zhang, J.; Zhao, Q.; Feliciano, J.; Bisz, E.; Dziuk, B.; Lalancette, R.; Szostak, R.; Szostak, M. Pd–PEPPSI N-Heterocyclic Carbene Complexes from Caffeine: Application in Suzuki, Heck, and Sonogashira Reactions. Organometallics 2022, 41, 2281–2290. [Google Scholar] [CrossRef]
- Organ, M.G.; Çalimsiz, S.; Sayah, M.; Hoi, K.H.; Lough, A.J. Pd-PEPPSI-IPent: An Active, Sterically Demanding Cross-Coupling Catalyst and Its Application in the Synthesis of Tetra-Ortho-Substituted Biaryls. Angew. Chem. Int. Ed. 2009, 48, 2383–2387. [Google Scholar] [CrossRef]
- Zhang, X.; Wright, A.M.; DeYonker, N.J.; Hollis, T.K.; Hammer, N.I.; Webster, C.E.; Valente, E.J. Synthesis, Air Stability, Photobleaching, and DFT Modeling of Blue Light Emitting Platinum CCC-N-Heterocyclic Carbene Pincer Complexes. Organometallics 2012, 31, 1664–1672. [Google Scholar] [CrossRef]
- Savka, R.; Plenio, H. Metal Complexes of Very Bulky N,N′-Diarylimidazolylidene N-Heterocyclic Carbene (NHC) Ligands with 2,4,6-Cycloalkyl Substituents. Eur. J. Inorg. Chem. 2014, 2014, 6246–6253. [Google Scholar] [CrossRef]
- Dinda, J.; Adhikary, S.D.; Roymahapatra, G.; Nakka, K.K.; Santra, M.K. Synthesis, structure, electrochemistry and cytotoxicity studies of Ru(II) and Pt(II)–N-heterocyclic carbene complexes of CNC-pincer ligand. Inorg. Chim. Acta 2014, 413, 23–31. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Kjaer, K.S.; Fredin, L.A.; Chabera, P.; Harlang, T.; Canton, S.E.; Lidin, S.; Zhang, J.X.; Lomoth, R.; Bergquist, K.E.; et al. A Heteroleptic Ferrous Complex with Mesoionic Bis(1,2,3-triazol-5-ylidene) Ligands: Taming the MLCT Excited State of Iron(II). Chem.-Eur. J. 2015, 21, 3628–3639. [Google Scholar] [CrossRef]
- Santini, C.; Marinelli, M.; Pellei, M. Boron-Centered Scorpionate-Type NHC-Based Ligands and Their Metal Complexes. Eur. J. Inorg. Chem. 2016, 2016, 2312–2331. [Google Scholar] [CrossRef]
- Balinge, K.R.; Bhagat, P.R. Palladium–N-heterocyclic carbene complexes for the Mizoroki–Heck reaction: An appraisal. Comptes Rendus Chim. 2017, 20, 773–804. [Google Scholar] [CrossRef]
- Tendera, L.; Schaub, T.; Krahfuss, M.J.; Kuntze-Fechner, M.W.; Radius, U. Large vs. Small NHC Ligands in Nickel(0) Complexes: The Coordination of Olefins, Ketones and Aldehydes at [Ni(NHC) 2]. Eur. J. Inorg. Chem. 2020, 2020, 3194–3207. [Google Scholar] [CrossRef]
- Stoppa, V.; Scattolin, T.; Bevilacqua, M.; Baron, M.; Graiff, C.; Orian, L.; Biffis, A.; Menegazzo, I.; Roverso, M.; Bogialli, S.; et al. Mononuclear and dinuclear gold(i) complexes with a caffeine-based di(N-heterocyclic carbene) ligand: Synthesis, reactivity and structural DFT analysis. New J. Chem. 2021, 45, 961–971. [Google Scholar] [CrossRef]
- Scheele, U.J.; John, M.; Dechert, S.; Meyer, F. Pyrazole-Bridged NHC Ligands and Their Dimetallic (Allyl)palladium Complexes. Eur. J. Inorg. Chem. 2008, 2008, 373–377. [Google Scholar] [CrossRef]
- Luo, F.-T.; Lo, H.-K. Short synthesis of bis-NHC-Pd catalyst derived from caffeine and its applications to Suzuki, Heck, and Sonogashira reactions in aqueous solution. J. Organomet. Chem. 2011, 696, 1262–1265. [Google Scholar] [CrossRef]
- Lo, H.-K.; Luo, F.-T. Synthesis of PS-supported NHC-Pd Catalyst Derived from Theobromine and its Applications in Suzuki-Miyaura Reaction. J. Chin. Chem. Soc. 2012, 59, 394–398. [Google Scholar] [CrossRef]
- Zhu, H.; Shen, Y.; Deng, Q.; Chen, J.; Tu, T. Pd(NHC)-catalyzed alkylsulfonylation of boronic acids: A general and efficient approach for sulfone synthesis. Chem. Commun. 2017, 53, 12473–12476. [Google Scholar] [CrossRef] [PubMed]
- Astakhov, A.V.; Khazipov, O.V.; Chernenko, A.Y.; Pasyukov, D.V.; Kashin, A.S.; Gordeev, E.G.; Khrustalev, V.N.; Chernyshev, V.M.; Ananikov, V.P. A New Mode of Operation of Pd-NHC Systems Studied in a Catalytic Mizoroki–Heck Reaction. Organometallics 2017, 36, 1981–1992. [Google Scholar] [CrossRef]
- Scattolin, T.; Caligiuri, I.; Canovese, L.; Demitri, N.; Gambari, R.; Lampronti, I.; Rizzolio, F.; Santo, C.; Visentin, F. Synthesis of new allyl palladium complexes bearing purine-based NHC ligands with antiproliferative and proapoptotic activities on human ovarian cancer cell lines. Dalton Trans. 2018, 47, 13616–13630. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, D.; Lin, Z.; Wu, W.; Jiang, H. Recent advances in NHC–palladium catalysis for alkyne chemistry: Versatile synthesis and applications. Org. Chem. Front. 2021, 8, 3502–3524. [Google Scholar] [CrossRef]
- Danopoulos, A.A.; Tulloch, A.A.D.; Winston, S.; Eastham, G.; Hursthouse, M.B. Chelating and ‘pincer’ dicarbene complexes of palladium; synthesis and structural studies. Dalton Trans. 2003, 1009–1015. [Google Scholar] [CrossRef]
- Tulloch, A.A.D.; Danopoulos, A.A.; Tizzard, G.J.; Coles, S.J.; Hursthouse, M.B.; Hay-Motherwell, R.S.; Motherwell, W.B. Chiral 2,6-lutidinyl-biscarbene complexes of palladium. Chem. Commun. 2001, 14, 1270–1271. [Google Scholar] [CrossRef]
- O’Brien, C.J.; Kantchev, E.A.B.; Valente, C.; Hadei, N.; Chass, G.A.; Lough, A.; Hopkinson, A.C.; Organ, M.G. Easily Prepared Air- and Moisture-Stable Pd–NHC (NHC=N-Heterocyclic Carbene) Complexes: A Reliable, User-Friendly, Highly Active Palladium Precatalyst for the Suzuki–Miyaura Reaction. Eur. J. Chem. 2006, 12, 4743–4748. [Google Scholar] [CrossRef]
- Tu, T.; Malineni, J.; Dötz, K.H. A Novel Pyridine-Bridged Bis-benzimidazolylidene Pincer Palladium Complex: Synthesis and Catalytic Properties. Adv. Synth. Catal. 2008, 350, 1791–1795. [Google Scholar] [CrossRef]
- Scattolin, T.; Giust, S.; Bergamini, P.; Caligiuri, I.; Canovese, L.; Demitri, N.; Gambari, R.; Lampronti, I.; Rizzolio, F.; Visentin, F. Palladacyclopentadienyl complexes bearing purine-based N-heterocyclic carbenes: A new class of promising antiproliferative agents against human ovarian cancer. Appl. Organomet. Chem. 2019, 33, e4902. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, B.; Yan, X.; Guo, S. Palladium pincer-type complexes and zwitterionic sulfur adducts of pyridine-bridged bis(1,2,3-triazolin-5-ylidenes): Syntheses, characterizations and catalytic applications. Dalton Trans. 2018, 47, 528–537. [Google Scholar] [CrossRef]
- Peris, E.; Mata, J.; Loch, J.A.; Crabtree, R.H. A Pd complex of a tridentate pincer CNC bis-carbene ligand as a robust homogenous Heck catalyst. Chem. Commun. 2001, 2, 201–202. [Google Scholar] [CrossRef]
- Warner, J.; Anastas, P. Green chemistry theory & practice: Principle 1. From improving what is to inventing what could be. Abstr. Pap. Am. Chem. Soc. 2018, 255. [Google Scholar]
- Petrucci, R.; Feroci, M.; Mattiello, L.; Chiarotto, I. Xanthine Scaffold: Available Synthesis Routes to Deliver Diversity by Derivatization. Mini-Rev. Org. Chem. 2021, 18, 27–42. [Google Scholar] [CrossRef]
- Dockendorff, B.; Holman, D.A.; Christian, G.D.; Ruzicka, J. Automated solid phase extraction of theophylline by sequential injection on renewable column. Anal. Commun. 1998, 35, 357–359. [Google Scholar] [CrossRef]
- Vandeponseele, A.; Draye, M.; Piot, C.; Chatel, G. Study of Influential Parameters of the Caffeine Extraction from Spent Coffee Grounds: From Brewing Coffee Method to the Waste Treatment Conditions. Clean Technol. 2021, 3, 335–350. [Google Scholar] [CrossRef]
- Machmudah, S.; Kitada, K.; Sasaki, M.; Goto, M.; Munemasa, J.; Yamagata, M. Simultaneous Extraction and Separation Process for Coffee Beans with Supercritical CO2 and Water. Ind. Eng. Chem. Res. 2011, 50, 2227–2235. [Google Scholar] [CrossRef]
- Saldaña, M.D.A.; Mohamed, R.S.; Baer, M.G.; Mazzafera, P. Extraction of Purine Alkaloids from Maté (Ilex paraguariensis) Using Supercritical CO2. J. Agric. Food Chem. 1999, 47, 3804–3808. [Google Scholar] [CrossRef]
- Schütz, J.; Herrmann, W.A. Purine-based carbenes at rhodium and iridium. J. Organomet. Chem. 2004, 689, 2995–2999. [Google Scholar] [CrossRef]
- Wetmore, C.M.; Scholes, D.; LaCroix, A.Z.; Ott, S.M.; Ichikawa, L. Longitudinal analysis of the association between habitual caffeine intake and bone mineral density among women aged 14 to 40. Am. J. Epidemiol. 2006, 163, S146. [Google Scholar] [CrossRef]
- Kascatan-Nebioglu, A.; Melaiye, A.; Hindi, K.; Durmus, S.; Panzner, M.J.; Hogue, L.A.; Mallett, R.J.; Hovis, C.E.; Coughenour, M.; Crosby, S.D.; et al. Synthesis from Caffeine of a Mixed N-Heterocyclic Carbene−Silver Acetate Complex Active against Resistant Respiratory Pathogens. J. Med. Chem. 2006, 49, 6811–6818. [Google Scholar] [CrossRef]
- Kascatan-Nebioglu, A.; Panzner, M.J.; Tessier, C.A.; Cannon, C.L.; Youngs, W.J. N-Heterocyclic carbene–silver complexes: A new class of antibiotics. Coord. Chem. Rev. 2007, 251, 884–895. [Google Scholar] [CrossRef]
- Wetmore, C.M.; Ichikawa, L.; LaCroix, A.Z.; Ott, S.M.; Scholes, D. Association between caffeine intake and bone mass among young women: Potential effect modification by depot medroxyprogesterone acetate use. Osteoporosis Int. 2008, 19, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Landaeta, V.R.; Rodríguez-Lugo, R.E.; Rodríguez-Arias, E.N.; Coll-Gómez, D.S.; González, T. Studies on the coordination chemistry of methylated xanthines and their imidazolium salts. Part 1: Benzyl derivatives. Transit. Met. Chem. 2010, 35, 165–175. [Google Scholar] [CrossRef]
- Hu, J.J.; Bai, S.-Q.; Yeh, H.H.; Young, D.J.; Chi, Y.; Hor, T.S.A. N-heterocyclic carbene Pt(ii) complexes from caffeine: Synthesis, structures and photoluminescent properties. Dalton Trans. 2011, 40, 4402. [Google Scholar] [CrossRef] [PubMed]
- Makhloufi, A.; Frank, W.; Ganter, C. Converting Caffeine to Electronically Different N-Heterocyclic Carbenes with a Hypoxanthine Backbone. Organometallics 2012, 31, 7272–7277. [Google Scholar] [CrossRef]
- Bertrand, B.; Stefan, L.; Pirrotta, M.; Monchaud, D.; Bodio, E.; Richard, P.; Le Gendre, P.; Warmerdam, E.; De Jager, M.H.; Groothuis, G.M.M.; et al. Caffeine-Based Gold(I) N-Heterocyclic Carbenes as Possible Anticancer Agents: Synthesis and Biological Properties. Inorg. Chem. 2014, 53, 2296–2303. [Google Scholar] [CrossRef]
- Mohamed, H.A.; Lake, B.R.M.; Laing, T.; Phillips, R.M.; Willans, C.E. Synthesis and anticancer activity of silver(i)–N-heterocyclic carbene complexes derived from the natural xanthine products caffeine, theophylline and theobromine. Dalton Trans. 2015, 44, 7563–7569. [Google Scholar] [CrossRef]
- Connahan, L.E.; Ott, C.A.; Barry, V.W. Effects of Caffeine on Blood Pressure during a Maximal Test in Active College-aged Females. Med. Sci. Sport Exer. 2015, 47, 887. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Che, C.-M.; Ott, I. Caffeine derived platinum(II) N-heterocyclic carbene complexes with multiple anti-cancer activities. J. Organomet. Chem. 2015, 782, 37–41. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Muenzner, J.K.; Abu el Maaty, M.A.; Karge, B.; Schobert, R.; Wölfl, S.; Ott, I. A multi-target caffeine derived rhodium(i) N-heterocyclic carbene complex: Evaluation of the mechanism of action. Dalton Trans. 2016, 45, 13161–13168. [Google Scholar] [CrossRef]
- Szadkowska, A.; Staszko, S.; Zaorska, E.; Pawłowski, R. A theophylline based copper N-heterocyclic carbene complex: Synthesis and activity studies in green media. RSC Adv. 2016, 6, 44248–44253. [Google Scholar] [CrossRef]
- Valdés, H.; Canseco-González, D.; Germán-Acacio, J.M.; Morales-Morales, D. Xanthine based N-heterocyclic carbene (NHC) complexes. J. Organomet. Chem. 2018, 867, 51–54. [Google Scholar] [CrossRef]
- Meng, D.; Li, D.; Ollevier, T. Recyclable iron(ii) caffeine-derived ionic salt catalyst in the Diels–Alder reaction of cyclopentadiene and α,β-unsaturated N-acyl-oxazolidinones in dimethyl carbonate. RSC Adv. 2019, 9, 21956–21963. [Google Scholar] [CrossRef]
- Gajare, S.P.; Bansode, P.A.; Patil, P.V.; Patil, A.D.; Pore, D.M.; Sonawane, K.D.; Dhanavade, M.J.; Khot, V.M.; Rashinkar, G.S. Anticancer, Antibacterial and Hyperthermia Studies of a Caffeine-Based N-Heterocyclic Carbene Silver Complex Anchored on Magnetic Nanoparticles. Chemistryselect 2021, 6, 1958–1968. [Google Scholar] [CrossRef]
- Teng, Q.; Zhao, Y.; Lu, Y.; Liu, Z.; Chen, H.; Yuan, D.; Huynh, H.V.; Meng, Q. Synthesis, Characterization, and Catalytic Study of Caffeine-Derived N-heterocyclic Carbene Palladium Complexes. Organometallics 2022, 41, 161–168. [Google Scholar] [CrossRef]
- Francescato, G.; Silva, S.M.d.; Leitão, M.I.P.S.; Gaspar-Cordeiro, A.; Giannopoulos, N.; Gomes, C.S.B.; Pimentel, C.; Petronilho, A. Nickel N-heterocyclic carbene complexes based on xanthines: Synthesis and antifungal activity on Candida sp. Appl. Organomet. Chem. 2022, 36, e6687. [Google Scholar] [CrossRef]
- Bysewski, O.; Winter, A.; Liebing, P.; Schubert, U.S. Noble Metal Complexes of a Bis-Caffeine Containing NHC Ligand. Molecules 2022, 27, 4316. [Google Scholar] [CrossRef]
- Kascatan-Nebioglu, A.; Panzner, M.J.; Garrison, J.C.; Tessier, C.A.; Youngs, W.J. Synthesis and Structural Characterization of N-Heterocyclic Carbene Complexes of Silver(I) and Rhodium(I) from Caffeine. Organometallics 2004, 23, 1928–1931. [Google Scholar] [CrossRef]
- Mohammadi, E.; Movassagh, B. Polystyrene-resin supported N-heterocyclic carbene-Pd(II) complex based on plant-derived theophylline: A reusable and effective catalyst for the Suzuki-Miyaura cross-coupling reaction of arenediazonium tetrafluoroborate salts with arylboronic acids. J. Organomet. Chem. 2016, 822, 62–66. [Google Scholar] [CrossRef]
- Widegren, J.A.; Finke, R.G. A review of the problem of distinguishing true homogeneous catalysis from soluble or other metal-particle heterogeneous catalysis under reducing conditions. J. Mol. Catal. A Chem. 2003, 198, 317–341. [Google Scholar] [CrossRef]
- Chernyshev, V.M.; Astakhov, A.V.; Chikunov, I.E.; Tyurin, R.V.; Eremin, D.B.; Ranny, G.S.; Khrustalev, V.N.; Ananikov, V.P. Pd and Pt Catalyst Poisoning in the Study of Reaction Mechanisms: What Does the Mercury Test Mean for Catalysis? ACS Catal. 2019, 9, 2984–2995. [Google Scholar] [CrossRef]
- Manar, K.K.; Ren, P. Chapter Four—Recent progress on group 10 metal complexes of pincer ligands: From synthesis to activities and catalysis. In Advances in Organometallic Chemistry; Pérez, P.J., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 76, pp. 185–259. [Google Scholar]
- Maji, A.; Singh, O.; Singh, S.; Mohanty, A.; Maji, P.K.; Ghosh, K. Palladium-Based Catalysts Supported by Unsymmetrical XYC–1 Type Pincer Ligands: C5 Arylation of Imidazoles and Synthesis of Octinoxate Utilizing the Mizoroki–Heck Reaction. Eur. J. Inorg. Chem. 2020, 2020, 1596–1611. [Google Scholar] [CrossRef]
- Qian, H.; Yu, S.; Song, L.; Zhang, T.; Yin, Z.; Zhao, F.; Yang, J.; Wang, C. ONO pincer palladium (II) complexes featuring furoylhydrazone ligands: Synthesis, characterization and catalytic activity towards Suzuki–Miyaura coupling reaction. Appl. Organomet. Chem. 2019, 33, e5116. [Google Scholar] [CrossRef]
- Takenaka, K.; Uozumi, Y. An N-C-N Pincer Palladium Complex as an Efficient Catalyst Precursor for the Heck Reaction. Adv. Synth. Catal. 2004, 346, 1693–1696. [Google Scholar] [CrossRef]
- Littke, A.F.; Fu, G.C. Palladium-Catalyzed Coupling Reactions of Aryl Chlorides. Angew. Chem. Int. Ed. 2002, 41, 4176–4211. [Google Scholar] [CrossRef]
- Párkányi, C.; Boniface, C.; Aaron, J.-J.; Bulaceanu-MacNair, M.; Dakkouri, M. Theoretical and experimental dipole moments of purines. Collect. Czech. Chem. Commun. 2002, 67, 1109–1124. [Google Scholar] [CrossRef]
- Nielsen, D.J.; Cavell, K.J.; Skelton, B.W.; White, A.H. A pyridine bridged dicarbene ligand and its silver(I) and palladium(II) complexes: Synthesis, structures, and catalytic applications. Inorg. Chim. Acta 2002, 327, 116–125. [Google Scholar] [CrossRef]



| Entry | Solvent | Base | Temperature/°C | Yield/% |
|---|---|---|---|---|
| 1 | H2O | K2CO3 | 50 | 3 |
| 2 | H2O | K3PO4 | 50 | 7 |
| 3 | H2O | KOH | 50 | 8 |
| 4 | THF/H2O | K2CO3 | 50 | 80 |
| 5 | THF/H2O | K3PO4 | 50 | 76 |
| 6 | THF/H2O | KOH | 50 | 77 |
| 7 | THF/H2O | K2CO3 | 40 | 45 |
| 8 | THF/H2O | K2CO3 | 70 | 92 |
| 9 b | THF/H2O | K2CO3 | 40 | 19 |
| 10 b | THF/H2O | K2CO3 | 50 | 65 |
| 11 c | THF/H2O | K2CO3 | 50 | 0 |
| Entry | Compound | Yield/% |
|---|---|---|
| 1 |  | 0 a |
| 2 |  | 0 a |
| 3 |  | 0 a |
| 4 |  | 45 a |
| 5 |  | 19 a |
| 6 |  | 100 b |
| 7 |  | 100 c |
| 8 |  | 100 c |
| 9 b |  | 100 c |
| Entry | Solvent | Base | Temperature/°C | Yield/% |
|---|---|---|---|---|
| 1 | H2O | K2CO3 | 100 | 0 |
| 2 | H2O + Aliquot 336 | K2CO3 | 100 | 0 |
| 3 | DMAc | K2CO3 | 100 | 24 |
| 4 | NMP | K2CO3 | 100 | 36 |
| 5 | NMP | Net3 | 100 | 1 |
| 6 | NMP | Piperidine | 100 | 0 |
| 7 | NMP | K2CO3 | 100 | 0 b |
| Entry | Compound | Yield/% |
|---|---|---|
| 1 |  | 0 a |
| 2 |  | 0 a |
| 3 |  | 100 a, 95 b, 20 c |
| 4 |  | 31 a |
| 5 |  | 25 a |
| 6 |  | 100 c |
| 7 |  | 100 c |
| 8 |  | 100 c |
| 9 |  | 100 c |
| Entry | Solvent | Base | Temperature/°C | Yield/% |
|---|---|---|---|---|
| 1 | H2O | NEt3 | 80 | 0 |
| 2 | DMAc | NEt3 | 80 | 0 |
| 3 | THF | NEt3 | 80 | 0 |
| 4 | H2O | NEt3 | 100 | 0 |
| 5 | DMAc | NEt3 | 100 | 20 |
| 6 | THF | NEt3 | 100 | 6 |
| 7 | DMAc | NEt3 | 120 | 37 b |
| 8 | DMAc | DIPA | 100 | 0 c |
| 9 | DMAc | Piperidine | 100 | 73 |
| 10 | DMAc | Piperidine | 100 | 51 d |
| Entry | Compound | Yield/% |
|---|---|---|
| 1 |  | 22 c |
| 2 |  | 0 a |
| 3 |  | 31 c |
| 4 |  | 35 b |
| 5 |  | 12 b |
| 6 |  | 100 c |
| 7 |  | 100 c |
| 8 |  | 100 c |
| 9 |  | 100 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bysewski, O.; Winter, A.; Schubert, U.S. Catalysis of a Bis-Caffeine Palladium(II) NHC-Pincer Complex. Inorganics 2023, 11, 164. https://doi.org/10.3390/inorganics11040164
Bysewski O, Winter A, Schubert US. Catalysis of a Bis-Caffeine Palladium(II) NHC-Pincer Complex. Inorganics. 2023; 11(4):164. https://doi.org/10.3390/inorganics11040164
Chicago/Turabian StyleBysewski, Oliver, Andreas Winter, and Ulrich S. Schubert. 2023. "Catalysis of a Bis-Caffeine Palladium(II) NHC-Pincer Complex" Inorganics 11, no. 4: 164. https://doi.org/10.3390/inorganics11040164
APA StyleBysewski, O., Winter, A., & Schubert, U. S. (2023). Catalysis of a Bis-Caffeine Palladium(II) NHC-Pincer Complex. Inorganics, 11(4), 164. https://doi.org/10.3390/inorganics11040164