Exploring the Interaction of Pyridine-Based Chalcones with Trinuclear Silver(I) Pyrazolate Complex
Abstract
:1. Introduction
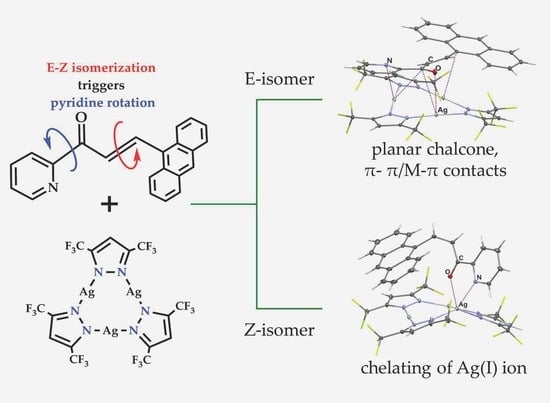
2. Results and Discussions
3. Materials and Methods
3.1. Physical Measurement and Instrumentation
3.2. Crystal Structure Determination
3.3. Computational Details
3.4. Synthesis and Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahmudov, K.T.; Kopylovich, M.N.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Non-covalent interactions in the synthesis of coordination compounds: Recent advances. Coord. Chem. Rev. 2017, 345, 54–72. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J.; Frontera, A. Not Only Hydrogen Bonds: Other Noncovalent Interactions. Crystals 2020, 10, 180. [Google Scholar] [CrossRef]
- Cornaton, Y.; Djukic, J.P. Noncovalent Interactions in Organometallic Chemistry: From Cohesion to Reactivity, a New Chapter. Acc. Chem. Res. 2021, 54, 3828–3840. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhen, X.; Wang, B.; Gao, X.; Ren, Z.; Wang, J.; Xie, Y.; Li, J.; Peng, Q.; Pu, K.; et al. The influence of the molecular packing on the room temperature phosphorescence of purely organic luminogens. Nat. Commun. 2018, 9, 840. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Yan, D. Hydrogen-Bonded Two-Component Ionic Crystals Showing Enhanced Long-Lived Room-Temperature Phosphorescence via TADF-Assisted Förster Resonance Energy Transfer. Adv. Funct. Mater. 2019, 29, 1807599. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Jin, W.J. Halogen bonding in room-temperature phosphorescent materials. Coord. Chem. Rev. 2020, 404, 213107. [Google Scholar] [CrossRef]
- McNaughton, D.A.; Fares, M.; Picci, G.; Gale, P.A.; Caltagirone, C. Advances in fluorescent and colorimetric sensors for anionic species. Coord. Chem. Rev. 2021, 427, 213573. [Google Scholar] [CrossRef]
- El Sayed Moussa, M.; Evariste, S.; Wong, H.L.; Le Bras, L.; Roiland, C.; Le Polles, L.; Le Guennic, B.; Costuas, K.; Yam, V.W.; Lescop, C. A solid state highly emissive Cu(i) metallacycle: Promotion of cuprophilic interactions at the excited states. Chem. Commun. 2016, 52, 11370–11373. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, K.; Teat, S.J.; Dey, G.; Shen, Z.; Wang, L.; O’Carroll, D.M.; Li, J. All-in-One: Achieving Robust, Strongly Luminescent and Highly Dispersible Hybrid Materials by Combining Ionic and Coordinate Bonds in Molecular Crystals. J. Am. Chem. Soc. 2017, 139, 9281–9290. [Google Scholar] [CrossRef]
- Baranov, A.Y.; Pritchina, E.A.; Berezin, A.S.; Samsonenko, D.G.; Fedin, V.P.; Belogorlova, N.A.; Gritsan, N.P.; Artem’ev, A.V. Beyond Classical Coordination Chemistry: The First Case of a Triply Bridging Phosphine Ligand. Angew. Chem. Int. Ed. Engl. 2021, 60, 12577–12584. [Google Scholar] [CrossRef]
- Rogovoy, M.I.; Berezin, A.S.; Samsonenko, D.G.; Artem’ev, A.V. Silver(I)-Organic Frameworks Showing Remarkable Thermo-, Solvato- And Vapochromic Phosphorescence As Well As Reversible Solvent-Driven 3D-to-0D Transformations. Inorg. Chem. 2021, 60, 6680–6687. [Google Scholar] [CrossRef] [PubMed]
- Demyanov, Y.V.; Sadykov, E.H.; Rakhmanova, M.I.; Novikov, A.S.; Bagryanskaya, I.Y.; Artem’ev, A.V. Tris(2-Pyridyl)Arsine as a New Platform for Design of Luminescent Cu(I) and Ag(I) Complexes. Molecules 2022, 27, 6059. [Google Scholar] [CrossRef] [PubMed]
- Housecroft, C.E.; Constable, E.C. TADF: Enabling luminescent copper(i) coordination compounds for light-emitting electrochemical cells. J. Mater. Chem. C Mater. 2022, 10, 4456–4482. [Google Scholar] [CrossRef] [PubMed]
- Yersin, H.; Czerwieniec, R.; Monkowius, U.; Ramazanov, R.; Valiev, R.; Shafikov, M.Z.; Kwok, W.-M.; Ma, C. Intersystem crossing, phosphorescence, and spin-orbit coupling. Two contrasting Cu(I)-TADF dimers investigated by milli- to micro-second phosphorescence, femto-second fluorescence, and theoretical calculations. Coord. Chem. Rev. 2023, 478, 214975. [Google Scholar] [CrossRef]
- Cui, Y.; Li, B.; He, H.; Zhou, W.; Chen, B.; Qian, G. Metal-Organic Frameworks as Platforms for Functional Materials. Acc. Chem. Res. 2016, 49, 483–493. [Google Scholar] [CrossRef]
- Lin, R.B.; Liu, S.Y.; Ye, J.W.; Li, X.Y.; Zhang, J.P. Photoluminescent Metal-Organic Frameworks for Gas Sensing. Adv. Sci. 2016, 3, 1500434. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Yang, M.; Chen, X.L.; Lu, C.Z. Bright bluish-green emitting Cu(I) complexes exhibiting efficient thermally activated delayed fluorescence. Dalton Trans. 2021, 50, 5171–5176. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Wang, S.-D.; Lv, J.-H.; Cao, K.-Z.; Liu, H.-Q.; Wang, F.; Lu, S.-Q. Novel nickel(II) coordination polymer based on a semi-rigid tricarboxylate acid ligand: Synthesis, structure, and fluorescence recognition of acetylacetone in aqueous media. J. Mol. Struct. 2022, 1247, 131317. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, J.; Guo, R.; Xu, J.; Fang, X.; Han, Y.-F. Cobalt phthalocyanine coordinated to pyridine-functionalized carbon nanotubes with enhanced CO2 electroreduction. Appl. Catal. B Environ. 2019, 251, 112–118. [Google Scholar] [CrossRef]
- Olivo, G.; Capocasa, G.; Del Giudice, D.; Lanzalunga, O.; Di Stefano, S. New horizons for catalysis disclosed by supramolecular chemistry. Chem. Soc. Rev. 2021, 50, 7681–7724. [Google Scholar] [CrossRef]
- Larionov, V.A.; Feringa, B.L.; Belokon, Y.N. Enantioselective “organocatalysis in disguise” by the ligand sphere of chiral metal-templated complexes. Chem. Soc. Rev. 2021, 50, 9715–9740. [Google Scholar] [CrossRef] [PubMed]
- Titov, A.A.; Filippov, O.A.; Epstein, L.M.; Belkova, N.V.; Shubina, E.S. Macrocyclic copper(I) and silver(I) pyrazolates: Principles of supramolecular assemblies with Lewis bases. Inorg. Chim. Acta 2018, 470, 22–35. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, H.; Xie, M.; Li, D. The pi-acidity/basicity of cyclic trinuclear units (CTUs): From a theoretical perspective to potential applications. Chem. Commun. 2019, 55, 7134–7146. [Google Scholar] [CrossRef]
- Zheng, J.; Lu, Z.; Wu, K.; Ning, G.H.; Li, D. Coinage-Metal-Based Cyclic Trinuclear Complexes with Metal-Metal Interactions: Theories to Experiments and Structures to Functions. Chem. Rev. 2020, 120, 9675–9742. [Google Scholar] [CrossRef]
- Dias, H.V.R.; Diyabalanage, H.V.K.; Rawashdeh-Omary, M.A.; Franzman, M.A.; Omary, M.A. Bright Phosphorescence of a Trinuclear Copper(I) Complex: Luminescence Thermochromism, Solvatochromism, and “Concentration Luminochromism”. J. Am. Chem. Soc. 2003, 125, 12072–12073. [Google Scholar] [CrossRef] [PubMed]
- Dias, H.V.; Diyabalanage, H.V.; Eldabaja, M.G.; Elbjeirami, O.; Rawashdeh-Omary, M.A.; Omary, M.A. Brightly phosphorescent trinuclear copper(I) complexes of pyrazolates: Substituent effects on the supramolecular structure and photophysics. J. Am. Chem. Soc. 2005, 127, 7489–7501. [Google Scholar] [CrossRef] [PubMed]
- Parasar, D.; Ponduru, T.T.; Noonikara-Poyil, A.; Jayaratna, N.B.; Dias, H.V.R. Acetylene and terminal alkyne complexes of copper(I) supported by fluorinated pyrazolates: Syntheses, structures, and transformations. Dalton Trans. 2019, 48, 15782–15794. [Google Scholar] [CrossRef]
- Titov, A.A.; Larionov, V.A.; Smol’yakov, A.F.; Godovikova, M.I.; Titova, E.M.; Maleev, V.I.; Shubina, E.S. Interaction of a trinuclear copper(I) pyrazolate with alkynes and carbon-carbon triple bond activation. Chem. Commun. 2019, 55, 290–293. [Google Scholar] [CrossRef]
- Yang, L.; Wang, L.; Lv, X.; Chen, J.H.; Wang, Y.; Yang, G. Complexation of triangular silver(I) or copper(I) nitropyrazolates with dibenzothiophenes having potential use in adsorptive desulfurization. Dalton Trans. 2021, 50, 2915–2927. [Google Scholar] [CrossRef]
- Larionov, V.A.; Stashneva, A.R.; Titov, A.A.; Lisov, A.A.; Medvedev, M.G.; Smol’yakov, A.F.; Tsedilin, A.M.; Shubina, E.S.; Maleev, V.I. Mechanistic study in azide-alkyne cycloaddition (CuAAC) catalyzed by bifunctional trinuclear copper(I) pyrazolate complex: Shift in rate-determining step. J. Catal. 2020, 390, 37–45. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, J.N.; Wang, T.; Wu, K.; Wei, R.J.; Lu, W.G.; Li, D. Phosphorescent Metal Rotaxane-like Bimetallic Ag/Au Clusters. J. Phys. Chem. C 2021, 125, 9400–9410. [Google Scholar] [CrossRef]
- Zhang, M.-M.; Dong, X.-Y.; Wang, Y.-J.; Zang, S.-Q.; Mak, T.C.W. Recent progress in functional atom-precise coinage metal clusters protected by alkynyl ligands. Coord. Chem. Rev. 2022, 453, 214315. [Google Scholar] [CrossRef]
- Morishima, Y.; Young, D.J.; Fujisawa, K. Structure and photoluminescence of silver(i) trinuclear halopyrazolato complexes. Dalton Trans. 2014, 43, 15915–15928. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Liu, Y.M.; Zhang, J.X.; Zhu, Y.Y.; Tang, M.S.; Ng, S.W.; Yang, G. Halogen-involving weak interactions manifested in the crystal structures of silver(I) or gold(I) 4-halogenated-3,5-diphenylpyrazolato trimers. Crystengcomm 2014, 16, 4987–4998. [Google Scholar] [CrossRef]
- Titov, A.A.; Guseva, E.A.; Filippov, O.A.; Babakhina, G.M.; Godovikov, I.A.; Belkova, N.V.; Epstein, L.M.; Shubina, E.S. The Role of Weak Interactions in Strong Intermolecular M...Cl Complexes of Coinage Metal Pyrazolates: Spectroscopic and DFT Study. J. Phys. Chem. A 2016, 120, 7030. [Google Scholar] [CrossRef]
- Zhan, S.Z.; Li, M.; Zheng, J.; Wang, Q.J.; Ng, S.W.; Li, D. Luminescent Cu4I4-Cu3(Pyrazolate)3 Coordination Frameworks: Postsynthetic Ligand Substitution Leads to Network Displacement and Entanglement. Inorg. Chem. 2017, 56, 13446–13455. [Google Scholar] [CrossRef]
- Zhan, S.Z.; Jiang, X.; Zheng, J.; Huang, X.D.; Chen, G.H.; Li, D. A luminescent supramolecular Cu2I2(NH3)2-sandwiched Cu3(pyrazolate)3 adduct as a temperature sensor. Dalton Trans. 2018, 47, 3679–3683. [Google Scholar] [CrossRef]
- Omary, M.A.; Rawashdeh-Omary, M.A.; Diyabalanage, H.V.; Dias, H.V. Blue phosphors of dinuclear and mononuclear copper(I) and silver(I) complexes of 3,5-bis(trifluoromethyl)pyrazolate and the related bis(pyrazolyl)borate. Inorg. Chem. 2003, 42, 8612. [Google Scholar] [CrossRef]
- Yang, C.; Elbjeirami, O.; Gamage, C.S.P.; Dias, H.V.R.; Omary, M.A. Luminescence enhancement and tuning via multiple cooperative supramolecular interactions in an ion-paired multinuclear complex. Chem. Commun. 2011, 47, 7434–7436. [Google Scholar] [CrossRef]
- Titov, A.A.; Smol’yakov, A.F.; Baranova, K.F.; Filippov, O.A.; Shubina, E.S. Synthesis, structures and photophysical properties of phosphorus-containing silver 3,5-bis(trifluoromethyl)pyrazolates. Mendeleev Commun. 2018, 28, 387–389. [Google Scholar] [CrossRef]
- Titov, A.A.; Filippov, O.A.; Smol’yakov, A.F.; Baranova, K.F.; Titova, E.M.; Averin, A.A.; Shubina, E.S. Dinuclear CuI and AgI Pyrazolates Supported with Tertiary Phosphines: Synthesis, Structures, and Photophysical Properties. Eur. J. Inorg. Chem. 2019, 2019, 821–827. [Google Scholar] [CrossRef]
- Titov, A.A.; Filippov, O.A.; Smol’yakov, A.F.; Averin, A.A.; Shubina, E.S. Synthesis, structures and luminescence of multinuclear silver(i) pyrazolate adducts with 1,10-phenanthroline derivatives. Dalton Trans. 2019, 48, 8410–8417. [Google Scholar] [CrossRef] [PubMed]
- Yakovlev, G.B.; Titov, A.A.; Smol’yakov, A.F.; Chernyadyev, A.Y.; Filippov, O.A.; Shubina, E.S. Tetranuclear Copper(I) and Silver(I) Pyrazolate Adducts with 1,1’-Dimethyl-2,2’-bibenzimidazole: Influence of Structure on Photophysics. Molecules 2023, 28, 1189. [Google Scholar] [CrossRef] [PubMed]
- Titov, A.A.; Smol’yakov, A.F.; Filippov, O.A. Heterobimetallic Silver(I) and Copper(I) pyrazolates supported with 1,1′-bis(diphenylphosphino)ferrocene. J. Organomet. Chem. 2021, 942, 121813. [Google Scholar] [CrossRef]
- Titov, A.A.; Filippov, O.A.; Guseva, E.A.; Smol’yakov, A.F.; Dolgushin, F.M.; Epstein, L.M.; Belsky, V.K.; Shubina, E.S. Role of basic sites of substituted ferrocenes in interaction with the trinuclear 3,5-bis(trifluoromethyl)pyrazolates: Thermodynamics and structure of complexes. RSC Adv. 2014, 4, 8350. [Google Scholar] [CrossRef]
- Titov, A.A.; Smol’yakov, A.F.; Godovikov, I.A.; Chernyadyev, A.Y.; Molotkov, A.P.; Loginov, D.A.; Filippov, O.A.; Belkova, N.V.; Shubina, E.S. The role of weak intermolecular interactions in photophysical behavior of isocoumarins on the example of their interaction with cyclic trinuclear silver(I) pyrazolate. Inorg. Chim. Acta 2022, 539, 121004. [Google Scholar] [CrossRef]
- Nongbri, S.L.; Das, B.; Rao, K.M. Isolation and spectral studies of water-soluble η5-cyclichydrocarbon rhodium and iridium complexes with pyridyl diketone analogues bonded through κ2-N∩O,κ4–N∩O, and κ3-N-C-N modes. J. Coord. Chem. 2012, 65, 875–890. [Google Scholar] [CrossRef]
- Zhang, H.-N.; Wang, J.; Hu, H.-M.; Yuan, F.; Song, J.; Xue, G.-L. Crystal Structures and Luminescent Properties of Two Zinc(II) Coordination Compounds with a β-Diketonate Derivative Ligand. Z. Anorg. Allg. Chem. 2013, 639, 1850–1854. [Google Scholar] [CrossRef]
- Balázs, L.B.; Tay, W.S.; Li, Y.; Pullarkat, S.A.; Leung, P.-H. Synthesis of Stereoprojecting, Chiral N-C(sp3)-E Type Pincer Complexes. Organometallics 2018, 37, 2272–2285. [Google Scholar] [CrossRef]
- Thomas, J.M.; Kuduvalli, S.S.; Anitha, T.S.; Sivasankar, C. Investigation of the CO releasing ability of azachalcone bound Mn(I) tricarbonyl complexes and their antiproliferative properties. Appl. Organomet. Chem. 2022, 36, 6896. [Google Scholar] [CrossRef]
- Alecu, I.M.; Zheng, J.; Zhao, Y.; Truhlar, D.G. Computational Thermochemistry: Scale Factor Databases and Scale Factors for Vibrational Frequencies Obtained from Electronic Model Chemistries. J. Chem. Theory Comput. 2010, 6, 2872–2887. [Google Scholar] [CrossRef]
- Constable, E.C.; Smith, D.R. Photoisomerisation of 9-anthrylsubstituted pyridyl enones. Tetrahedron 1996, 52, 935–940. [Google Scholar] [CrossRef]
- Titov, A.A.; Smol’yakov, A.F.; Filippov, O.A.; Godovikov, I.A.; Muratov, D.A.; Dolgushin, F.M.; Epstein, L.M.; Shubina, E.S. Supramolecular Design of the Trinuclear Silver(I) and Copper(I) Metal Pyrazolates Complexes with Ruthenium Sandwich Compounds via Intermolecular Metal−π Interactions. Cryst. Growth Des. 2017, 17, 6770–6779. [Google Scholar] [CrossRef]
- Duraimurugan, K.; Harikrishnan, M.; Madhavan, J.; Siva, A.; Lee, S.J.; Theerthagiri, J.; Choi, M.Y. Anthracene-based fluorescent probe: Synthesis, characterization, aggregation-induced emission, mechanochromism, and sensing of nitroaromatics in aqueous media. Environ. Res. 2021, 194, 110741. [Google Scholar] [CrossRef] [PubMed]
- Omary, M.A.; Rawashdeh-Omary, M.A.; Gonser, M.W.A.; Elbjeirami, O.; Grimes, T.; Cundari, T.R.; Diyabalanage, H.V.K.; Gamage, C.S.P.; Dias, H.V.R. Metal Effect on the Supramolecular Structure, Photophysics, and Acid−Base Character of Trinuclear Pyrazolato Coinage Metal Complexes. Inorg. Chem. 2005, 44, 8200–8210. [Google Scholar] [CrossRef] [PubMed]
- APEX II software Package; Bruker AXS Inc.: Madison, WI, USA, 2005.
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2011, 2, 73–78. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Li, G.D.; Mao, S.P.; Chai, J.D. Long-Range Corrected Hybrid Density Functionals with Improved Dispersion Corrections. J. Chem. Theory Comput. 2013, 9, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Rasika Dias, H.V.; Polach, S.A.; Wang, Z. Coinage metal complexes of 3,5-bis(trifluoromethyl)pyrazolate ligand: Synthesis and characterization of {[3,5-(CF3)2Pz]Cu}3 and {[3,5-(CF3)2Pz]Ag}3. J. Fluor. Chem. 2000, 103, 163–169. [Google Scholar] [CrossRef]









| ΔG298 | ν(C=O) (A) | ν(C=C) (A) | |
|---|---|---|---|
| anti-E-1 | 0.0 | 1682 (269) a | 1611 (573) b |
| anti-E-1′ | +2.7 | 1691 (278) a | 1613 (553) b |
| syn-E-1 | +2.1 | 1658 (700) | 1638 (118) |
| syn-E-1′ | +3.7 | 1671 (685) | 1634 (159) |
| anti-Z-1 | +2.8 | 1690 (287) a | 1637 (84); 1627 (5) |
| anti-Z-1′ | +5.2 | 1696 (303) a | 1637 (112); 1627 (4) |
| syn-Z-1 | +2.0 | 1671 (601) | 1632 (27); 1625 (11) |
| syn-Z-1′ | +2.7 | 1680 (591) | 1631 (26); 1624 (21) |
 | E-1(δ, ppm) | + [AgPz]3 (Δδ, ppm) | Z-1 (δ, ppm) | + [AgPz]3 (Δδ, ppm) | |
| C=O | 3 | 187.51 | −0.17 | 187.73 | +0.66 |
| Py | 4 | 154.34 | +0.04 | 154.29 | −0.59 |
| 5 | 148.63 | +0.62 | 148.27 | +0.19 | |
| 6 | 128.10 | +0.15 | 126.69 | −0.10 | |
| 7 | 136.37 | +0.33 | 136.14 | −0.07 | |
| 8 | 122.82 | +0.48 | 122.30 | +0.21 | |
| C=C | 1 | 140.85 | +0.08 | 140.55 | −0.65 |
| 2 | 130.63 | −0.6 | 131.17 | −0.27 | |
| Anthracene | 9 | 131.32 | −0.26 | 131.78 | −0.15 |
| 10 | 128.66 | −0.16 | 128.30 | +0.08 | |
| 11 | 126.07 | +0.55 | 125.75 | −0.26 | |
| 12 | 125.12 | −0.11 | 124.81 | +0.31 | |
| 13 | 125.67 | −0.18 | 125.22 | +0.27 | |
| 14 | 130.00 | −0.18 | 128.66 | +0.03 | |
| 15 | 129.85 | −0.24 | 128.63 | −0.04 | |
| 16 | 126.20 | +0.42 | 125.94 | +0.13 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olbrykh, A.; Titov, A.; Smol’yakov, A.; Filippov, O.; Shubina, E.S. Exploring the Interaction of Pyridine-Based Chalcones with Trinuclear Silver(I) Pyrazolate Complex. Inorganics 2023, 11, 175. https://doi.org/10.3390/inorganics11040175
Olbrykh A, Titov A, Smol’yakov A, Filippov O, Shubina ES. Exploring the Interaction of Pyridine-Based Chalcones with Trinuclear Silver(I) Pyrazolate Complex. Inorganics. 2023; 11(4):175. https://doi.org/10.3390/inorganics11040175
Chicago/Turabian StyleOlbrykh, Arina, Aleksei Titov, Alexander Smol’yakov, Oleg Filippov, and Elena S. Shubina. 2023. "Exploring the Interaction of Pyridine-Based Chalcones with Trinuclear Silver(I) Pyrazolate Complex" Inorganics 11, no. 4: 175. https://doi.org/10.3390/inorganics11040175
APA StyleOlbrykh, A., Titov, A., Smol’yakov, A., Filippov, O., & Shubina, E. S. (2023). Exploring the Interaction of Pyridine-Based Chalcones with Trinuclear Silver(I) Pyrazolate Complex. Inorganics, 11(4), 175. https://doi.org/10.3390/inorganics11040175