Method for Decontamination of Toxic Aluminochrome Catalyst Sludge by Reduction of Hexavalent Chromium
Abstract
:1. Introduction
2. Literature Review
3. Results and Discussion
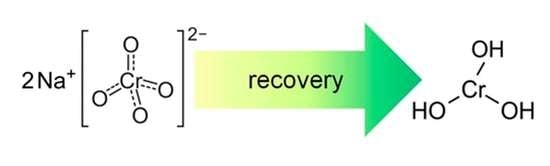
3.1. Recovery of Hexavalent Chromium in the Liquid Phase of Catalyst Sludge
3.2. Recovery of Hexavalent Chromium in Catalyst Slurry
- Sodium thiosulfate (Na2S2O3) showed a negative result for the reduction of Cr (VI) to Cr (III) in a neutral medium. The concentration of Cr (VI) in the solution in the temperature range of 50–85 °C did not change.
- Iron sulfate (anhydrous) in the reduction of Cr (VI) to Cr (III) in a neutral medium showed an average result, with an efficiency of about 30%. The reduction process proceeded without the use of additional precipitators, since the reduced chromium already precipitated out as Cr(OH)3 in the process of reduction. Moreover, during the reduction with ferrous sulfate, in addition to chromium hydroxide, iron hydroxide precipitated out, which was an undesirable effect, since these precipitates must be separated later.
- The reduction of Cr (VI) to Cr (III) in a neutral medium in the presence of sodium sulfite proceeded with 100% efficiency without the use of additional precipitators. However, during the reduction with sodium sulfite, the solutions acquired a strongly alkaline environment, which should be neutralized. In addition, this interaction produced a byproduct in the form of sulfur dioxide, which must be captured and disposed of.
- The recovery of the liquid phase of the catalyst sludge with sodium metabisulfite (Na2S2O5) completely proceeded without the formation of byproducts, as with the other reagents previously described. Its effect in a neutral environment led to a conversion of up to 100% of hexavalent chromium to the trivalent state, with the formation of a precipitate in the form of chromium hydroxide, without the use of additional reagents. Therefore, sodium metabisulfite was chosen for the reduction of hexavalent chromium in the catalyst slurry.
- The experiments on the conversion of hexavalent chromium to the trivalent state in the catalyst slurry showed that the reduction process in the presence of sodium metabisulfite Na2S2O5 can be directly carried out without the separation of the solid and liquid phases. Complete reduction took place at 85 °C after 10 min of interaction with the reducing agent.
4. Materials and Methods
- −
- separation of the liquid phase of the slurry from the solid phase;
- −
- analysis of the solid and liquid phases before reduction;
- −
- slurry liquid-phase recovery;
- −
- filtration of liquid phase after recovery;
- −
- analysis of liquid phase after recovery;
- −
- selection of the most effective reagent for recovery of the liquid phase (Na2SO3, FeSO4, Na2S2O3, and Na2S2O5);
- −
- pulp reduction carried out with the selected reagent;
- −
- determination of the chemical composition of the chromium content in the liquid and solid phases.
4.1. Separation of the Liquid Phase of the Pulp
4.2. Analysis of the Obtained Phases
4.3. Recovering the Liquid Phase of the Slurry
4.4. Phase Analysis after Recovery
4.5. Conducting Pulp Recovery with the Selected Reagent
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bekmukhamedov, G.E. Modified Silicon Dioxide Aluminochrome Catalyst for Isobutane Dehydrogenation. Kazan (Volga Region) Federal University, Kazan, Russia, 2015. [Google Scholar]
- Kudinova, A.A.; Poltoratckaya, M.E.; Gabdulkhakov, R.R.; Litvinova, T.E.; Rudko, V.A. Parameters influence establishment of the petroleum coke genesis on the structure and properties of a highly porous carbon material obtained by activation of KOH. J. Porous Mater. 2022, 29, 1599–1616. [Google Scholar] [CrossRef]
- Nasifullina, A.I.; Starkov, M.K.; Gabdulkhakov, R.R.; Rudko, V.A. Petroleum coking additive-raw material component for metallurgical coke production. Part 2. Experimental studies of obtaining a petroleum coking additive. CIS Iron Steel Rev. 2022, 24, 9–16. [Google Scholar] [CrossRef]
- Pakhomov, N.A.; Kashkin, V.N.; Molchanov, V.V.; Noskov, A.S. Dehydrogenation of C2-C4 paraffins on Cr2O3/Al2O3 catalysts. Gas Chem. 2008, 154, 66–69. [Google Scholar]
- Bekmukhamedov, G.E.; Morozov, V.I.; Tuktarov, R.R.; Bukharov, M.S.; Egorova, S.R.; Lamberov, A.A.; Yakhvarov, D.G. Electronic interaction between Cr(Ⅲ) ions in chromia-alumina catalysts for light alkane dehydrogenation. J. Phys. Chem. Solids 2022, 167, 110778. [Google Scholar] [CrossRef]
- Krylov, O.V. Heterogeneous Catalysis: Textbook for Universities; Academkniga: Moscow, Russia, 2004; p. 679. [Google Scholar]
- Konoplin, R.; Kondrasheva, N. Difficulties in the industrial introduction of new effective hydrodesulfurization catalysts in the Russian Federation. E3S Web Conf. 2021, 266, 02016. [Google Scholar] [CrossRef]
- Dhal, B.; Thatoi, H.N.; Das, N.N.; Pandeya, B.D. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review. J. Hazard. Mater. 2013, 250–251, 272–291. [Google Scholar] [CrossRef]
- Kamaludeen, S.P.B.; Megharaj, M.; Juhasz, A.L.; Sethunathan, N.; Naidu, R. Chromium-Microorganism Interactions in Soils: Remediation Implications. Rev. Environ. Contam. Toxicol. 2003, 178, 93–164. [Google Scholar] [CrossRef]
- Chernobrovin, V.P. State of the chrome industry in Russia. Bull. SUSU Metall. Ser. 2017, 17, 44–48. [Google Scholar]
- Chrysochoou, M.; Johnston, C.P. Reduction of chromium (VI) in saturated zone sediments by calcium polysulfide and nanoscale zerovalent iron derived from green tea extract. State Art Pract. Geotech. Eng. 2012, 2012, 3959–3967. [Google Scholar]
- Lebedev, A.B.; Shuiskaya, V.S. Influence of composition and cooling rate of alumocalcium slag on its crumblability. Izv. Ferr. Met. 2022, 65, 806–813. [Google Scholar] [CrossRef]
- Lurie, Y.Y. Chemical Analysis of Industrial Wastewater; Chemistry: Moscow, Russia, 1974; 448p. [Google Scholar]
- Lebedev, A.B.; Musinova, P.V. Formation of the strength of pelletized multiphase dicalcium silicate sinter. Chernye Met. 2022, 40–46. [Google Scholar] [CrossRef]
- Shrivastava, R.; Upreti, R.K.; Chaturvedi, U.C. Various cells of the immune system and intestine differ in their capacity to reduce hexavalent chromium. FEMS Immunol. Med. Microbiol. 2003, 38, 65–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yemane, M.; Chandravanshi, B.S.; Wondimu, T. Levels of essential and nonessential metals in leaves of the tea plant (Camellia sinensis L.) and soil of Wushwush farms, Ethiopia. Food Chem. 2008, 107, 1236–1243. [Google Scholar]
- Pécou, E.; Maass, A.; Remenik, D.; Briche, J.; Gonzalez, M. A mathematical model for copper homeostasis in Enterococcus hirae. Math. Biosci. 2006, 203, 222–239. [Google Scholar] [CrossRef]
- Petrov, G.V.; Schneerson, J.M.; Andreev, Y.V. Extraction of platinum metals during processing of chromite ores from dunite massifs. Notes Min. Inst. 2018, 231, 281–286. [Google Scholar]
- Petrov, G.V.; Graver, T.N.; Tikhonov, O.N.; Belenkiy, A.M.; Boduen, A.Y. Study of Russian chromite platinum-metal massifs. Zap. Mines Inst. 2006, 169, 173–177. [Google Scholar]
- Ying, Z.; Ren, X.; Li, J.; Wu, G.; Wei, Q. Recovery of chromium(VI) in wastewater using solvent extraction with amide. Hydrometallurgy 2020, 196, 105440. [Google Scholar] [CrossRef]
- Dai, L.; Ding, J.; Liu, Y.; Wu, X.; Chen, L.; Chen, R.; Van der Bruggen, B. Recovery of Cr(VI) and removal of cationic metals from chromium slag using a modified bipolar membrane system. J. Membr. Sci. 2021, 639, 119772. [Google Scholar] [CrossRef]
- Kumar, A.; Thakur, A.; Panesar, P. Extraction of hexavalent chromium by environmentally benign green emulsion liquid membrane using tridodecyamine as an extractant. J. Ind. Eng. Chem. 2019, 70, 394–401. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, H.; Zhang, M.; Chen, R.; Chen, X.; Zheng, X.; Jin, Y. Cr(VI) recovery from chromite ore processing residual using an enhanced electrokinetic process by bipolar membranes. J. Membr. Sci. 2018, 566, 190–196. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, H.; Liu, Y.; Chen, R.; Qian, Q.; Van der Bruggen, B. Cr(III) recovery in form of Na2CrO4 from aqueous solution using improved bipolar membrane electrodialysis. J. Membr. Sci. 2020, 604, 118097. [Google Scholar] [CrossRef]
- Ye, Z.; Yin, X.; Chen, L.; He, X.; Lin, Z.; Liu, C.; Ning, S.; Wang, X.; Wei, Y. An integrated process for removal and recovery of Cr(VI) from electroplating wastewater by ion exchange and reduction–precipitation based on a silica-supported pyridine resin. J. Clean. Prod. 2019, 236, 117631. [Google Scholar] [CrossRef]
- Lu, J.; Liu, Z.; Wu, Z.; Liu, W.; Yang, C. Synergistic effects of binary surfactant mixtures in the removal of Cr(VI) from its aqueous solution by foam fractionation. Sep. Purif. Technol. 2020, 237, 116346. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, Q.; Gao, B.; Li, M.; Liu, C.; Qiu, Y. Removal of hexavalent chromium by biochar supported nZVI composite: Batch and fixed-bed column evaluations, mechanisms, and secondary contamination prevention. Chemosphere 2019, 217, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Wang, Y.; Tian, X.; Jiang, Z.; Deng, F.; Tao, Y.; Jiang, Q.; Wang, L.; Zhang, Y. KOH-activated porous biochar with high specific surface area for adsorptive removal of chromium (VI) and naphthalene from water: Affecting factors, mechanisms and reusability exploration. J. Hazard. Mater. 2020, 401, 123292. [Google Scholar] [CrossRef] [PubMed]
- Janik, P.; Zawisza, B.; Talik, E.; Sitko, R. Selective adsorption and determination of hexavalent chromium ions using graphene oxide modified with amino silanes. Microchim. Acta 2018, 185, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; An, S.; Lee, J.; Ghosh, A.; Zhong, M.; Fortner, J.D. Photoactive Polyethylenimine-Coated Graphene Oxide Composites for Enhanced Cr(VI) Reduction and Recovery. ACS Appl. Mater. Interfaces 2021, 13, 28027–28035. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Z.; Mei, J.; Hu, Q.; Chang, S.; Hong, Q.; Yang, S. Outstanding performance of sulfurated titanomaghemite (Fe2TiO5) for hexavalent chromium removal: Sulfuration promotion mechanism and its application in chromium resource recovery. Chemosphere 2022, 287, 132360. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Bidgoli, N.S.S.; Issaabadi, Z.; Ghavamifar, Z.; Baran, T.; Luque, R. Hibiscus rosasinensis L. aqueous extract-assisted valorization of lignin: Preparation of magnetically reusable Pd NPs@Fe3O4-lignin for Cr(VI) reduction and Suzuki-Miyaura reaction in eco-friendly media. Int. J. Biol. Macromol. 2020, 148, 265–275. [Google Scholar] [CrossRef]
- Fang, W.; Jiang, X.; Luo, H.; Geng, J. Synthesis of graphene/SiO2@polypyrrole nanocomposites and their application for Cr(VI) removal in aqueous solution. Chemosphere 2018, 197, 594–602. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Y.; Wang, J.; Wang, X.; Liu, B.; Wang, Y. In-situ remediation of hexavalent chromium contaminated groundwater and saturated soil using stabilized iron sulfide nanoparticles. J. Environ. Manag. 2019, 231, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Aigbe, U.O.; Osibote, O.A. A review of hexavalent chromium removal from aqueous solutions by sorption technique using nanomaterials. J. Environ. Chem. Eng. 2020, 8, 104503. [Google Scholar] [CrossRef]
- Shao, Q.; Xu, C.; Wang, Y.; Huang, S.; Zhang, B.; Huang, L.; Fan, D.; Tratnyek, P.G. Dynamic interactions between sulfidated zerovalent iron and dissolved oxygen: Mechanistic insights for enhanced chromate removal. Water Res. 2018, 135, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Azeez, N.A.; Dash, S.S.; Gummadi, S.N.; Deepa, V.S. Nano-remediation of toxic heavy metal contamination: Hexavalent chromium [Cr(VI)]. Chemosphere 2021, 266, 129204. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fan, G.; Zhu, X.; Li, H.; Li, Y.; Li, H.; Xu, X. The remediation of hexavalent chromium-contaminated soil by nanoscale zero-valent iron supported on sludge-based biochar. J. Soils Sediments 2023, 23, 1607–1616. [Google Scholar] [CrossRef]
- Golovina, V.V.; Eremina, A.O.; Sobolev, A.A.; Chesnokov, N.V. Extraction of chromium from aqueous solutions by porous materials based on logging waste of local wood raw materials (bark and wood chips). J. Sib. Fed. Univ. Ser. Chem. 2017, 10, 186–205. [Google Scholar] [CrossRef]
- Bagauva, A.I.; Stepanova, S.V.; Shaikhiev, I.G. Study of extracts from wood waste (sawdust oak bark) to remove chromium (VI) ions from the model solutions. Bull. Kazan Technol. Univ. 2011, 14, 74–79. [Google Scholar]
- Cherdchoo, W.; Nithettham, S.; Charoenpanich, J. Removal of Cr(VI) from synthetic wastewater by adsorption onto coffee ground and mixed waste tea. Chemosphere 2019, 221, 758–767. [Google Scholar] [CrossRef]
- Vilardi, G.; Ochando-Pulido, J.M.; Verdone, N.; Stoller, M.; Di Palma, L. On the removal of hexavalent chromium by olive stones coated by iron-based nanoparticles: Equilibrium study and chromium recovery. J. Clean. Prod. 2018, 190, 200–210. [Google Scholar] [CrossRef]
- Zorkina, O.V. Methods for converting hexavalent chromium to trivalent form by organic reducing agent. Proc. Penza State Pedagog. Univ. Named V.G. Belinsky 2011, 25, 690–696. [Google Scholar]
- Singh, P.; Itankar, N.; Patil, Y. Biomanagement of hexavalent chromium: Current trends and promising perspectives. J. Environ. Manag. 2020, 279, 111547. [Google Scholar] [CrossRef]
- Yogeshwaran, V.; Priya, A.K. Removal of Hexavalent Chromium (Cr(Ⅵ)) Using Different Natural Adsorbents—A Review. J. Chromatogr. Sep. Tech. 2017, 8, 2–6. [Google Scholar]
- Petrov, G.V.; Kalashnikova, M.I.; Fokina, S.B. Laws of behavior of selenium and chromium in oxidation-reduction processes during hydrometallurgical processing of solid-phase products of rhenium extraction. Proc. Min. Univ. 2016, 220, 601–606. [Google Scholar]
- Tsybulskaya, O.N.; Ksenik, T.V.; Kisel, A.A.; Yudakov, A.A.; Perfiliev, A.V.; Chirikov, A.Y. Decontamination of chromium-containing electroplating waste. New Technol. 2015, 4, 104–112. [Google Scholar]
- Karataev, O.R.; Kudryavtseva, E.S.; Mingazetdinov, I.H. Wastewater treatment from hexavalent chromium ions. Bull. Kazan Technol. Univ. 2014, 2, 52–54. [Google Scholar]
- Klimova, O.V. Processes and Apparatus Design of Wastewater Treatment from Chromium (VI) Ions by Carbon Adsorbents. Ph.D. Thesis, Irkutsk National Research Technical University, Irkutsk, Russia, 2015. [Google Scholar]
- Cespón-Romero, R.; Yebra-Biurrun, M.; Bermejo-Barrera, M. Preconcentration and speciation of chromium by the determination of total chromium and chromium(III) in natural waters by flame atomic absorption spectrometry with a chelating ion-exchange flow injection system. Anal. Chim. Acta 1996, 327, 37–45. [Google Scholar] [CrossRef]
- Torkmahalleh, M.A.; Lin, L.; Holsen, T.M.; Rasmussen, D.H.; Hopke, P.K. The Impact of Deliquescence and pH on Cr Speciation in Ambient PM Samples. Aerosol Sci. Technol. 2021, 46, 690–696. [Google Scholar] [CrossRef]
- Lee, T.J.; Kim, H.J. Interfering elements on determination of hexavalent chromium in papermaterials with UV-vis spectrophotometry. Nord. Pulp Pap. Res. J. 2021, 37, 130–137. [Google Scholar] [CrossRef]
- Korostelev, P.P. Titrimetric and Gravimetric Analysis in Metallurgy: Handbook. 1985. Available online: https://www.chem.msu.ru/rus/teaching/analyt/korostelev/all.pdf (accessed on 28 March 2023).
- Elanieva, S.I. Physico-chemical methods of reducing the aggressiveness of spent electrolytes by transferring CR (Ⅵ) into CR (Ⅲ) Proceedings of PSPU. Sect. Young Sci. 2008, 6, 174–178. [Google Scholar]
- Barrera-Díaz, C.E.; Lugo-Lugo, V.; Bilyeu, B. A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J. Hazard. Mater. 2012, 223–224, 1–12. [Google Scholar] [CrossRef]





| Name of Specimen and Research Instrument | Concentration, % wt. | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | SiO2 | K2O | CaO | TiO2 | Cr2O3 | Fe2O3 | ZnO | ZrO2 | |
| Solid phase of catalyst sludge (initial) (Shimadzu) | 72.46 | 11.61 | 2.49 | 0.18 | 0.23 | 11.74 | 0.21 | 0.01 | 0.23 |
| Solid phase of catalyst sludge (initial) (ISP MS) | 70.20 | 8.8 | 1.90 | 0.30 | 0.23 | 13.5 | 0.31 | - | - |
| Solid phase of catalyst sludge (initial) (microanalysis: Tescan Vega 3) | 71.8 | 10.08 | 2.94 | 0.35 | - | 13.6 | 0.27 | - | - |
| Experiment Number | Ratio of the Reducing Agent’s Mass to the Mass of the Liquid Phase of the Catalyst Slurry, g/g | Name of Reducing Agent | Process Temperature, °C | Process Time, min | Cr(VI) Removal Efficiency, % | Concentration of Cr+6 in the Liquid Phase before Reduction, g/L | Concentration of Cr+6 in the Liquid Phase after Recovery, g/L | pH of the Liquid Phase before Recovery | pH of the Liquid Phase after Recovery |
|---|---|---|---|---|---|---|---|---|---|
| Reduction with sodium sulfite (neutral medium) | |||||||||
| 1 | 0.0060 | Na2SO3 | 50 | 30 | 20 | 1.205 | 1.113 | 7 | 11.7–12.5 |
| 2 | 75 | 98 | Mehee 0.005 | ||||||
| 3 | 85 | 100 | 0 | ||||||
| 4 | 50 | 60 | 68 | 0.390 | |||||
| 5 | 75 | 98 | Mehee 0.005 | ||||||
| 6 | 85 | 100 | 0 | ||||||
| 7 | 50 | 120 | 78 | 0.265 | |||||
| 8 | 75 | 100 | 0 | ||||||
| 9 | 85 | 100 | 0 | ||||||
| 10 | 0.0037 | 50 | 60 | 7 | 1.105 | ||||
| 11 | 75 | 12 | 0.64 | ||||||
| 12 | 85 | 46 | 0.65 | ||||||
| 13 | 0.0019 | 75 | 10 | 0.89 | |||||
| Recovery with ferrous sulfate (neutral medium) | |||||||||
| 14 | 0.0060 | FeSO4 | 50 | 60 | 31.10 | 1.205 | 0.375 | 7 | 2.5–3.0 |
| 15 | 75 | 33.61 | 0.405 | ||||||
| 16 | 85 | 30.29 | 0.365 | ||||||
| Recovery with sodium thiosulfate (neutral medium) | |||||||||
| 17 | 0.0060 | Na2S2O3 | 50 | 60 | No sludge | 1.205 | 1.160 | 7 | 8.8 |
| 18 | 75 | 1.070 | |||||||
| 19 | 85 | 1.085 | |||||||
| Reduction with sodium metabisulphite (neutral medium) | |||||||||
| 20 | 0.0035 | Na2S2O5 | 50 | 60 | 47 | 1.205 | 0.64 | 7 | 8.8 |
| 21 | 85 | 65 | 52 | 0.58 | |||||
| 22 | 85 | 120 | 55 | 0.54 | |||||
| 23 | 50 | 420 | 57 | 0.52 | |||||
| 24 | 0.0070 | 50 | 15 | 0 | 1.20 | ||||
| 25 | 85 | 60 | 100 | 0 | |||||
| Duration of Experiment, Days | Concentration of Total Chromium in the Liquid Phase, g/L |
|---|---|
| 0 | 1.21 |
| 2 | 1.10 |
| 3 | 0.92 |
| 4 | 0.89 |
| 5 | 0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyagay, I.; Zubkova, O.; Zubakina, M.; Sizyakov, V. Method for Decontamination of Toxic Aluminochrome Catalyst Sludge by Reduction of Hexavalent Chromium. Inorganics 2023, 11, 284. https://doi.org/10.3390/inorganics11070284
Pyagay I, Zubkova O, Zubakina M, Sizyakov V. Method for Decontamination of Toxic Aluminochrome Catalyst Sludge by Reduction of Hexavalent Chromium. Inorganics. 2023; 11(7):284. https://doi.org/10.3390/inorganics11070284
Chicago/Turabian StylePyagay, Igor, Olga Zubkova, Margarita Zubakina, and Viktor Sizyakov. 2023. "Method for Decontamination of Toxic Aluminochrome Catalyst Sludge by Reduction of Hexavalent Chromium" Inorganics 11, no. 7: 284. https://doi.org/10.3390/inorganics11070284
APA StylePyagay, I., Zubkova, O., Zubakina, M., & Sizyakov, V. (2023). Method for Decontamination of Toxic Aluminochrome Catalyst Sludge by Reduction of Hexavalent Chromium. Inorganics, 11(7), 284. https://doi.org/10.3390/inorganics11070284