ZnO–Doped CaO Binary Core–Shell Catalysts for Biodiesel Production via Mexican Palm Oil Transesterification
Abstract
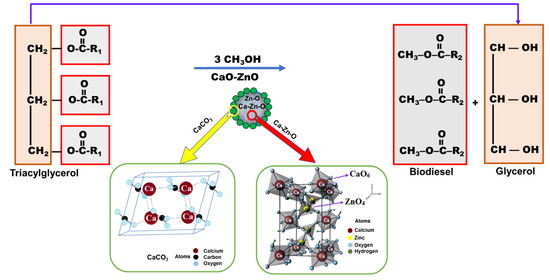
:1. Introduction
2. Results and Discussion
2.1. Phase Composition and XRD Analysis
2.2. FTIR Spectroscopy Analysis
2.3. Raman Spectroscopy
2.4. XPS Analysis
2.5. Surface Basicity
2.6. Morphological Features and AFM and TEM Observations
2.7. Palm Oil Transesterification Reactions
2.7.1. Thermal Treatment Effect
2.7.2. Catalyst Stability
2.7.3. FTIR Characterization of Biodiesel
2.8. Measurement of Biodiesel Indexes
3. Experimental Section
3.1. Reagents
3.2. Catalyst Preparation
3.3. Catalyst Characterization Techniques
3.4. Biodiesel Production, Purification, and Characterization
3.5. Ca2+ Solubility Measurement
3.6. Analyses of Biodiesel Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glîsić, S.; Lukic, I.; Skala, D. Biodiesel synthesis at high pressure and temperature: Analysis of energy consumption on industrial scale. Bioresour. Technol. 2009, 100, 6347–6354. [Google Scholar] [CrossRef]
- Ngamcharussrivichai, C.; Totarat, P.; Bunyakiat, K. Ca and Zn mixed oxide as a heterogeneous base catalyst for transesterification of palm kernel oil. Appl. Catal. A Gen. 2008, 341, 77–85. [Google Scholar] [CrossRef]
- Yan, S.; Di Maggio, C.; Mohan, S.; Kim, M.; Salley, S.O.; Ng, K.S. Advancements in Heterogeneous Catalysis for Biodiesel Synthesis. Top. Catal. 2010, 53, 721–736. [Google Scholar] [CrossRef]
- Xie, W.; Yang, X.; Fan, M. Biodiesel production by transesterification using tetraalkylammonium hydroxides immobilized onto SBA–15 as a solid catalyst. Renew. Energy 2015, 80, 230–237. [Google Scholar] [CrossRef]
- López–Granados, M.; Zafra–Poves, M.D.; Martín–Alonso, D.; Mariscal, R.; Cabello–Galisteo, F.; Moreno–Tost, R.; Santamaria, J.; Fierro, J.L.G. Biodiesel from sunflower oil by using activated calcium oxide. Appl. Catal. B 2007, 73, 317–326. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Abdul Aziz, A.R.; Sulaiman, N.M.N. The effects of water on biodiesel production and refining technologies: A review. Renew. Sustain. Energy Rev. 2012, 16, 3456–3470. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel production via non-catalytic SCF method and biodiesel fuel characteristics. Energy Convers. Manag. 2006, 47, 2271–2282. [Google Scholar] [CrossRef]
- Ma, F.; Clements, I.D.; Hanna, M.A. The effects of catalyst, free fatty acids, and water on transesterification of beef tallow. Trans. ASAE 1998, 41, 1261–1264. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Banković Ilić, B.; Miladinović, M.R.; Stamenković, O.S.; Veljković, V.B. Application of nano CaO–based catalysts in biodiesel synthesis. Renew. Sustain. Energy Rev. 2017, 72, 746–760. [Google Scholar] [CrossRef]
- Liu, X.; He, H.; Wang, Y.; Zhu, S. Transesterification of soybean oil to biodiesel using SrO as a solid base catalyst. Catal. Commun. 2007, 8, 1107–1111. [Google Scholar] [CrossRef]
- López Granados, M.; Martín Alonso, D.; Sadaba, I.; Mariscal, R.; Ocon, P. Leaching and homogeneous contribution in liquid phase reaction catalyzed by solids: The case of triglycerides methanolysis using CaO. Appl. Catal. B 2009, 89, 265–272. [Google Scholar] [CrossRef]
- Albuquerque, M.C.G.; Jiménez Urbistondo, J.; Santamaría González, J.; Mérida Robles, J.M.; Monreno Tost, R.; Rodrígue Castellón, E. CaO supported on mesoporous silica SBA–15 as basic catalysts for transesterification reactions. Appl. Catal. A 2008, 334, 35–43. [Google Scholar] [CrossRef]
- Dias, J.M.; Alvim Ferraz, M.C.M.; Almeida, M.F.; Díaz, J.D.M.; Pola, M.S.; Utrilla, J.R. Biodiesel production using calcium manganese oxide as catalyst and different raw materials. Energy Convers. Manag. 2013, 65, 647–653. [Google Scholar] [CrossRef]
- Dai, Y.-M.; Li, Y.-Y.; Lin, J.-H.; Kao, I.-H.; Lin, Y.-J.; Chen, C.-C. Applications of M2ZrO2 (M = Li, Na, K) composite as a catalyst for biodiesel production. Fuel 2021, 286, 119392. [Google Scholar] [CrossRef]
- Dai, Y.-M.; Li, Y.-Y.; Lin, J.-H.; Chen, B.Y.; Chen, C.-C. One-pot synthesis of acid-base bifunctional catalysts for biodiesel production. J. Environ. Manag. 2021, 299, 113592. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Yang, Z.; Chun, H. Catalysis properties of lithium–doped ZnO catalyst used for biofuel productions. Ind. Eng. Chem. Res. 2007, 46, 7942–7949. [Google Scholar] [CrossRef]
- Bournay, L.; Casanave, D.; Delfort, B.; Hillion, G.; Chodorge, J.A. New heterogeneous process for biodiesel production: A way to improve the quality and the value of the crude glycerin produced by biodiesel plants. Catal. Today 2005, 106, 190–192. [Google Scholar] [CrossRef]
- Thitsartarn, W.; Kawi, S. An active and stable CaO–CeO2 catalyst for Transesterification of oil to biodiesel. Green Chem. 2011, 13, 3423–3430. [Google Scholar] [CrossRef]
- Chen, G.; Shan, R.; Li, S.; Shi, J. A biomimetic silicification approach to synthesize CaO–SiO2 catalyst for the transesterification of palm oil into biodiesel. Fuel 2015, 153, 48–55. [Google Scholar] [CrossRef]
- Chansiriwat, W.; Wantala, K.; Khunphonoi, R.; Khemthong, P.; Suwannaruang, T.; Rood, S.C. Enhancing the catalytic performance of calcium-based catalyst derived from gypsum waste for renewable light fuel production through a pyrolysis process: A study on the effect of magnesium content. Chemosphere 2022, 292, 133516. [Google Scholar] [CrossRef]
- Chansiriwat, W.; Chotwatcharanurak, L.; Khumta, W.; Suwannaruang, T.; Shahmoradi, B.; Kumsaen, T.; Wantala, K. Biofuel production from waste cooking oil by catalytic reaction over Thai dolomite under atmospheric pressure: Effect of calcination temperatures. Eng. Appl. Sci. Res. 2021, 48, 102–111. [Google Scholar] [CrossRef]
- Albuquerque, M.C.G.; Azevedo, D.S.C.; Cavalgante, C.L.; Santamaría González, J.; Mérida Robles, J.M.; Moreno Tost, R.; Rodríguez Castellón, E.; Jiménez López, A.; Maireles Torres, P. Transesterification of ethyl butyrate with methanol using MgO/CaO catalysts. J. Mol. Catal. A 2009, 300, 19–24. [Google Scholar] [CrossRef]
- Woranuch, W.; Ngaosuwan, K.; Kiatkittipong, W.; Wongsawaeng, D.; Appamana, W.; Powell, J.; Rokhum, S.L.; Assabumrungrat, S. Fine-tuned fabrication parameters of CaO catalyst pellets for transesterification of palm oil to biodiesel. Fuel 2022, 323, 124356. [Google Scholar] [CrossRef]
- Kocík, J.; Hájek, M.; Troppová, I. The factors influencing stability of Ca–Al mixed oxides as a possible catalyst for biodiesel production. Fuel Process. Technol. 2015, 134, 297–302. [Google Scholar] [CrossRef]
- Wu, X.; Kang, M.; Zhao, N.; Wei, W.; Sun, Y. Dimethyl carbonate synthesis over ZnO–CaO bi-functional catalysts. Catal. Commun. 2014, 46, 46–50. [Google Scholar] [CrossRef]
- Margaretha, Y.Y.; Prastyo, S.H.; Ayucitra, A.; Ismadji, S. Calcium oxide from Pomacea sp. shell as a catalyst for biodiesel production. J. Energy Environ. Eng. 2012, 3, 33. [Google Scholar] [CrossRef]
- Xavier, C.S.; Sczancoski, J.C.; Cavalcante, L.S.; Paiva Santos, C.O.; Li, M.S. A new processing method of CaZn2(OH)6·2H2O powders: Photoluminescence and growth mechanism. Solid State Sci. 2009, 11, 2173–2179. [Google Scholar] [CrossRef]
- Lavalley, J.C. Infrared spectrometric studies of the surface basicity of metal oxides and zeolites using adsorbed probe molecules. Catal. Today 1996, 27, 377–401. [Google Scholar] [CrossRef]
- Fernández Carrasco, L.; Torrens Martín, D.; Morales, L.M.; Martínez Ramírez, S. Infrared Spectroscopy in the Analysis of Building and Construction Materials; Tech Open Publisher: London, UK, 2012. [Google Scholar] [CrossRef]
- Genge, M.J.; Jones, A.P.; David Price, G. An Infrared and Raman study of carbonate glasses: Implications for the structure of carbonatite magmas. Geochim. Cosmochim. Acta 1995, 59, 927–937. [Google Scholar] [CrossRef]
- Yang, C.C.; Chen, W.C.; Wang, C.L.; Wu, C.Y. Study the effect of conductive fillers on a secondary Zn electrode based on ball-milled ZnO and Ca(OH)2 mixture powders. J. Power Sources 2007, 172, 435–445. [Google Scholar] [CrossRef]
- Rieder, K.; Weinstein, B.; Cardona, M. Measurement and Comparative Analysis of the Second–Order Raman Spectra of the Alkaline-Earth Oxides with a NaCl Structure. Phys. Rev. B 1972, 8, 4780–4786. [Google Scholar] [CrossRef]
- Schmida, T.; Dariz, P. Shedding light onto the spectra of lime: Raman and luminescence bands of CaO, Ca(OH)2 and CaCO3. J. Raman Spectrosc. 2015, 46, 141–146. [Google Scholar] [CrossRef]
- Seehra, M. Comment on the Raman study of the thermal transformation of calcium hydroxide. J. Solid State Chem. 1986, 63, 344–345. [Google Scholar] [CrossRef]
- Ni, M.; Ratner, B.D. Differentiation of Calcium Carbonate Polymorphs by Surface Analysis Techniques—An XPS and TOF-SIMS study. Surf. Interface Anal. 2008, 40, 1356–1361. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Radiman, S.; Daud, A.R.; Tabet, N.; Al-Douri, Y. XPS and optical studies of different morphologies of ZnO nanostructures prepared by microwave methods. Ceram. Int. 2013, 39, 2283–2292. [Google Scholar] [CrossRef]
- Alba Rubio, A.C.; Santamaría González, J.; Mérida Robles, J.M.; Moreno Tost, R.; Martıín Alonso, D.; Jiménez López, A.; Maireles Torres, P. Heterogeneous transesterification processes by using CaO supported on zinc oxide as basic catalysts. Catal. Today 2010, 149, 281–287. [Google Scholar] [CrossRef]
- Lee, J.; Chung, J.; Lim, S. Improvement of optical properties of postannealed ZnO nanorods. Phys. E 2010, 42, 2143–2146. [Google Scholar] [CrossRef]
- Das, J.; Pradhan, S.K.; Sahu, S.R.; Mishra, D.K.; Sarangi, S.N.; Nayak, B.B.; Verma, S.; Roul, B.K. MicrO–Raman and XPS studies of pure ZnO ceramics. Phys. B 2010, 405, 2492–2497. [Google Scholar] [CrossRef]
- Doyle, C.S.; Kendelewicz, T.; Carrier, X.; Brown, G.E. The interaction of carbon dioxide with single crystal CaO (100) surfaces. Surf. Rev. Lett. 1999, 6, 1247–1254. [Google Scholar] [CrossRef]
- Weng, X.; Cui, Y.; Shaikhutdinov, S.; Freund, H.J. CO2 Adsorption on CaO(001): Temperature-Programmed Desorption and Infrared Study. J. Phys. Chem. C 2019, 123, 1880–1887. [Google Scholar] [CrossRef]
- Raouf, F.; Taghizadeh, M.; Yousef, M. Influence of CaO–ZnO supplementation as a secondary catalytic bed on the oxidative coupling of methane. React. Kinet. Mech. Catal. 2014, 112, 227–240. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, X.; Chen, X.; Sun, D.; Yu, X. Direct FTIR analysis of free fatty acids in edible oils using disposable polyethylene film. Food Anal. Methods 2015, 8, 857–863. [Google Scholar] [CrossRef]
- Rabelo, S.N.; Ferraz, V.P.; Oliveira, L.S.; Franca, A.S. FTIR Analysis for quantification of fatty acid methyl esters in biodiesel produced by microwave-assisted transesterification. Int. J. Environ. Sci. Dev. 2015, 6, 964–969. [Google Scholar] [CrossRef]
- Muik, B.; Lendl, B.; Molina, A.; Pérez, L.; Ayora, M.J. Determination of oil and water content in olive pomace using near infrared and Raman spectrometry. A comparative study. Anal. Bioanal. Chem. 2004, 379, 35–41. [Google Scholar] [CrossRef]
- Shapaval, V.; Brandenburg, J.; Blomqvist, J.; Tafintseva, V.; Passoth, V.; Sandgren, M.; Kohler, A. Biochemical profiling, prediction of total lipid content and fatty acid profile in oleaginous yeasts by FTIR spectroscopy. Biotechnol. Biofuels 2019, 12, 140. [Google Scholar] [CrossRef]
- Núñez, F.; Chen, L.; Wang, J.A.; Flores, S.O.; Salmones, J.; Arellano, U.; Noreña, L.E.; Tzompantzi, F. Bifunctional Co3O4/ZSM-5 Mesoporous Catalysts for Biodiesel Production via Esterification of Unsaturated Omega-9 Oleic Acid. Catalysts 2022, 12, 900. [Google Scholar] [CrossRef]
- Murguía–Ortiz, D.; Cordova, I.; Manriquez, M.E.; Ortiz-Islas, E.; Cabrera-Sierra, R.; Contreras, J.L.; Alcántar-Vázquez, B.; TrejO–Rubio, M. Vázquez-Rodríguez, J.T.; Castro, L.V. Na-CaO/MgO dolomites used as heterogeneous catalysts in canola oil transesterification for biodiesel production. Mater. Lett. 2021, 291, 129587. [Google Scholar] [CrossRef]
- Hu, S.; Guan, Y.; Wang, Y.; Han, H. NanO–magnetic catalyst KF/CaO–Fe3O4 for biodiesel production. Appl. Energy 2011, 88, 2685–2690. [Google Scholar] [CrossRef]
- Knothe, G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process. Technol. 2004, 86, 1059–1070. [Google Scholar] [CrossRef]
- Knothe, G. “Designer” biodiesel: Optimizing fatty ester composition to improve fuel properties. Energy Fuel 2008, 22, 1358–1364. [Google Scholar] [CrossRef]
- Hammond, E.G.; Lundberg, W.O. Molar refraction, molar volume and refractive index of fatty acid esters and related compounds in the liquid state. J. Am. Oil Chem. Soc. 1954, 31, 427–432. [Google Scholar] [CrossRef]
- Tat, M.E.; Van Gerpen, J.H. The specific gravity of biodiesel and its blends with diesel fuel. J. Am. Oil Chem. Soc. 2000, 77, 115–119. [Google Scholar] [CrossRef]
- Tekac, V.; Cibulka, I.; Holub, R. PVT properties of liquids and liquid mixtures: A review of the experimental methods and the literature data. Fluid Phase Equilib. 1985, 19, 133–149. [Google Scholar] [CrossRef]
- Pratas, M.J.; Freitas, S.; Oliveira, M.B.; Monteiro, S.C.; Lima, A.S.; Coutinho, J.A.P. Densities and viscosities of fatty acid methyl and ethyl esters. J. Chem. Eng. Data 2010, 55, 3983–3990. [Google Scholar] [CrossRef]
- Knothe, G.; Matheaus, A.C.; Ryan, T.W. Cetane numbers of branched and straight chain fatty esters determined in an ignition quality tester. Fuel 2003, 82, 971–975. [Google Scholar] [CrossRef]
- Gelbard, G.; Brès, O.; Vargas, R.M.; Vielfaure, F.; Schuchardt, U.F. 1H nuclear magnetic resonance determination of the yield of the transesterification of rapeseed oil with methanol. J. Am. Oil Chem. Soc. 1995, 72, 1239–1241. [Google Scholar] [CrossRef]
- Ramírez Verduzco, L.F.; Rodríguez Rodríguez, J.E.; Jaramillo Jacob, A.R. Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel 2012, 91, 102–111. [Google Scholar] [CrossRef]
- Bamgboyel, A.I.; Hansen, A.C. Prediction of cetane number of biodiesel fuel from the fatty acid methyl ester (FAME) composition. Int. Agrophysics 2008, 22, 21–29. [Google Scholar]









| Catalysts | ZnO Concentration (mol%) | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) | Surface Basicity (µmol/g) |
|---|---|---|---|---|---|
| CaO | 0 | 85.5 | 0.378 | 17.7 | 10.8 |
| CaO–ZnO–5% | 5 | 108.0 | 0.465 | 17.2 | 10.4 |
| CaO–ZnO–10% | 10 | 44.8 | 0.366 | 14.5 | 8.6 |
| CaO–ZnO–15% | 15 | 13.5 | 0.068 | 20.1 | 7.4 |
| CaO–ZnO–20% | 20 | 12.6 | 0.056 | 21.3 | 5.4 |
| Catalyst | Conversion (%) (without Thermal Treatment) | Conversion (%) (with Thermal Treatment) |
|---|---|---|
| CaO–ZnO–0% | 97.5 | 98.8 |
| CaO–ZnO–5% | 97.1 | 97.0 |
| CaO–ZnO–10% | 94.1 | 96.8 |
| CaO–ZnO–15% | 79.3 | 96.9 |
| CaO–ZnO–20% | 70.2 | 96.8 |
| Catalyst | Methanol Ca2+ (mg/L) | Methanol/Glycerol Mixture (4:1) Ca2+ (mg/L) | Palm Oil Ca2+ (mg/L) |
|---|---|---|---|
| CaO | 121.7 | 101.9 | 7.2 |
| CaO–ZnO–5% | 47.2 | 49.6 | 5.5 |
| CaO–ZnO–10% | 34.6 | 32.6 | 0 |
| CaO–ZnO–15% | 31.0 | 34.0 | 0 |
| CaO–ZnO–20% | 31.7 | 35.3 | 0 |
| Catalysts | Feedstocks | Alcohol/Oil Ratio | Catalyst/Oil (wt%) | Reaction Temperature (°C) | Reaction Time (h) | Yield or Conversion (%) | Refs. |
|---|---|---|---|---|---|---|---|
| CaO–ZnO (10mol% ZnO) | Palm oil + methanol | 4:1 | 2.2 | 60 | 3 | 96.8 | This work |
| CaO/γ-Al2O3 | Palm oil + methanol | 12:1 | 10 | 65 | 6 | 93.0 | [24] |
| CaO–SiO2 (2Si5Ca) | Palm oil + methanol | 15:1 | 9 | 65 | 8 | 80.1 | [20] |
| Na-CaO/MgO | Canola oil + methanol | 12:1 | 5 | 65 | 6 | 95.4 | [49] |
| CaO–CeO2 (1Ca1Ce-650) | palm oil + methanol | 20:1 | 5 | 85 | 10 | >90 | [19] |
| KF/CaO/Fe3O4 (Calcination at 600 °C) | Stillingia oil + methanol | 12:1 | 4 | 65 | 3 | 95 | [50] |
| Catalysts | Density (g/cm3) | Cetane Number | Average Molecular Weight (g/mol) | Refractive Index. |
|---|---|---|---|---|
| CaO | 0.8680 | 71.38 | 271.2 | 1.456 |
| CaO–ZnO–10% | 0.8753 | 67.43 | 269.6 | 1.442 |
| CaO–ZnO–15% | 0.8789 | 62.16 | 270.3 | 1.458 |
| Component | Mass Fraction (%) | Molar Fraction (%) | Molecular Mass (g/mol) |
|---|---|---|---|
| Tripalmitin | 47.1 | 45.0 | 807.5 |
| Triolein | 37.6 | 33.2 | 885.6 |
| Trilinolein | 11.5 | 10.1 | 879.6 |
| Palmitic acid | 3.8 | 12.7 | 256.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arenas-Quevedo, M.G.; Manríquez, M.E.; Wang, J.A.; Elizalde-Solís, O.; González-García, J.; Zúñiga-Moreno, A.; Chen, L.F. ZnO–Doped CaO Binary Core–Shell Catalysts for Biodiesel Production via Mexican Palm Oil Transesterification. Inorganics 2024, 12, 51. https://doi.org/10.3390/inorganics12020051
Arenas-Quevedo MG, Manríquez ME, Wang JA, Elizalde-Solís O, González-García J, Zúñiga-Moreno A, Chen LF. ZnO–Doped CaO Binary Core–Shell Catalysts for Biodiesel Production via Mexican Palm Oil Transesterification. Inorganics. 2024; 12(2):51. https://doi.org/10.3390/inorganics12020051
Chicago/Turabian StyleArenas-Quevedo, M. G., M. E. Manríquez, J. A. Wang, O. Elizalde-Solís, J. González-García, A. Zúñiga-Moreno, and L. F. Chen. 2024. "ZnO–Doped CaO Binary Core–Shell Catalysts for Biodiesel Production via Mexican Palm Oil Transesterification" Inorganics 12, no. 2: 51. https://doi.org/10.3390/inorganics12020051
APA StyleArenas-Quevedo, M. G., Manríquez, M. E., Wang, J. A., Elizalde-Solís, O., González-García, J., Zúñiga-Moreno, A., & Chen, L. F. (2024). ZnO–Doped CaO Binary Core–Shell Catalysts for Biodiesel Production via Mexican Palm Oil Transesterification. Inorganics, 12(2), 51. https://doi.org/10.3390/inorganics12020051