Luminescent Lanthanide Metal Organic Frameworks for cis-Selective Isoprene Polymerization Catalysis
Abstract
:1. Introduction
2. Results and Discussion


2.1. Mixed Ln-MOF Synthesis for Ln(form)3


2.2. Pure Lanthanide-Based MOFs
2.3. MIL-103(Nd) with Eu-Insertion
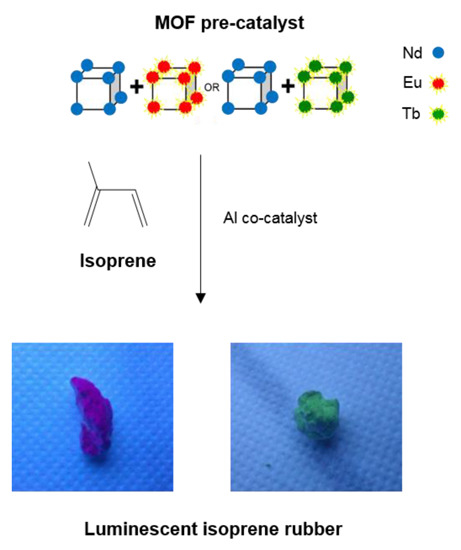
2.4. Elaboration of cis-Polyisoprene/MOF Composite Materials

2.4.1. Mixed Nd/Ln(form)3 MOF Polymerization (Ln = Eu, Tb)
| Run a | Ratio of Nd/Ln | Yield (%) | Mn (Ð) b | Selectivity (%) c |
|---|---|---|---|---|
| cis-/trans-/3,4- | ||||
| 1 d | 100% Nd | 27 | 64,900 (2.3) | 92/2/6 |
| 2 d | 5.14 | 46 | nd | 84/8/8 |
| 3 d | 1.59 | 29 | 34,400 (5.0) | 95/1/4 |
| 4 d | 0.49 | 11 | nd | 79/14/7 |
| 5 d | 100% Eu | 5 | - | - |
| 6 e | 3.00 | 40 | 77,600 (2.4) | 92/2/6 |
| 7 e | 1.00 | 36 | 49,700 (3.5) | 82/1/17 |
| 8 e | 0.33 | 35 | 35,600 (3.8) | 85/7/8 |
| 9 e | 100% Tb | 1 | nd | - |
2.4.2. Pure MOF-[Nd(form)3 + Ln(form)3] Polymerization (Ln = Eu, Tb)
| Run a | Ratio of Reagents | Time (h) | Yield (%) | Selectivity (%) b | Luminescence |
|---|---|---|---|---|---|
| Nd/Ln/MMAO/Isoprene | cis-/trans-/3,4- | ||||
| 10 | 1/10Eu/100/500 | 48 | 77 | 87/5/8 | yes |
| 11 c | 10/10Eu/100/500 | 48 | 78 | 93/2/5 | yes |
| 12 d | 50/50Eu/100/500 | 75 | 80 | 92/2/6 | yes |
| 13 d | 50/50Eu/100/500 | 336 | 89 | 86/4/10 | yes |
| 14 | 1/10Tb/100/500 | 48 | 77 | 70/4/26 | yes |
| 15 d | 50/50Tb/100/500 | 166 | 64 | 91/2/7 | yes |
| 16 d | 50/50Tb/100/500 | 336 | 85 | 89/6/5 | yes |
| 17 e | 1/92/500 | 48 | 17 | 88/1/11 | no |


2.4.3. Polymerization with MIL-103(Nd) with Eu-Inserted
3. Experimental Section
3.1. MOF Syntheses
3.1.1. Pure MOF Synthesis—Ln(form)3 (Ln = Nd, Eu, Tb)
3.1.2. Mixed Ln-MOF Synthesis
| Structure | Ln Ratio (Feed) | Ln Ratio (ICP-AES) |
|---|---|---|
| Ln(form)3 | 1Nd:1Eu | 1.59 |
| Ln(form)3 | 1Nd:3Eu | 0.49 |
| Ln(form)3 | 3Nd:1Eu | 5.14 |
| Ln(form)3 | 1Nd:1Tb | - |
| Ln(form)3 | 1Nd:3Tb | - |
| Ln(form)3 | 3Nd:1Tb | - |
3.2. Isoprene Polymerisation
3.3. MOF Characterization
3.3.1. Powder X-Ray Diffraction (PXRD)
3.3.2. Scanning Electron Microscopy (SEM)
3.3.3. ICP-AES
3.4. Polymer Material Characterization
3.4.1. Polymer Yield
3.4.2. NMR Analysis
3.4.3. Luminescence
3.4.4. Preparation of Polymer Films–Spin Coater
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Special Issue—Metal-Organic Frameworks. Available online: http://pubs.acs.org/toc/chreay/112/2 (accessed on 5 August 2015).
- Binnemans, K. Lanthanide-based luminescent hybrid materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal-organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Vitorino, M.; Devic, T.; Tromp, M.; Ferey, G.; Visseaux, M. Lanthanide metal-organic frameworks as Ziegler–Natta catalysts for the selective polymerisation of isoprene. Macromol. Chem. Phys. 2009, 210, 1923–1932. [Google Scholar] [CrossRef]
- Rodrigues, I.; Mihalcea, I.; Volkringer, C.; Loiseau, T.; Visseaux, M. Water-free neodymium 2,6-naphthalenedicarboxylates coordination complexes and their application as catalysts for isoprene polymerisation. Inorg. Chem. 2012, 51, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Escribano, P.; Julian-Lopez, B.; Planelles-Arago, J.; Cordoncillo, E.; Viana, B.; Sanchez, C. Photonic and nanobiophotonic properties of luminescent lanthanide-doped hybrid organic–inorganic materials. J. Mater. Chem. 2008, 18, 23–40. [Google Scholar] [CrossRef]
- Hou, S.; Chan, W.K. Preparation of functionalized polystyrene-block-polyisoprene copolymers and their luminescence properties. Macromolecules 2002, 35, 850–856. [Google Scholar] [CrossRef]
- Otsuka, T.; Chujo, Y. Highly stabilized luminescent polymer nanocomposites: Fluorescence emission from metal quinolate complexes with inorganic nanocrystals. J. Mater. Chem. 2010, 20, 10688–10695. [Google Scholar] [CrossRef]
- Yang, C.; Sun, Z.; Liu, L.; Zhang, L. Preparation and luminescence performance of rare earth agriculture-used light transformation composites. J. Mater. Sci. 2008, 43, 1681–1687. [Google Scholar] [CrossRef]
- Liu, F.; Carlos, L.D.; Ferreira, R.A.S.; Rocha, J.; Ferro, M.C.; Tourrette, A.; Quignard, F.; Robitzer, M. Synthesis, texture, and photoluminescence of lanthanide-containing chitosan-silica hybrids. J. Phys. Chem. B 2010, 114, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Agostini, G. Self-luminescent pneumatic tire. U.S. Patent S7234498 B2, 26 June 2004. [Google Scholar]
- Zuo, Y.; Lu, H.; Xue, L.; Wang, X.; Wu, L.; Feng, S. Polysiloxane-based luminescent elastomers prepared by thiol-ene “click” chemistry. Chem. Eur. J. 2014, 20, 12924–12932. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Li, J.; Lee, K.I.; Shao, S.; Hao, J.; Fei, B.; Xin, J.H. Reversible mechanochromism of a luminescent elastomer. Appl. Mater. Interfaces 2013, 5, 4625–4631. [Google Scholar] [CrossRef] [PubMed]
- Devic, T.; Wagner, V.; Guillou, N.; Vimont, A.; Haouas, M.; Pascolini, M.; Serre, C.; Marrot, J.; Daturi, M.; Taulelle, F.; et al. Synthesis and characterization of a series of porous lanthanide tricarboxylates. Microporous Mesoporous Mater. 2011, 140, 25–33. [Google Scholar] [CrossRef]
- An, J.; Shade, C.M.; Chengelis-Czegan, D.A.; Petoud, S.; Rosi, N.L. Zinc-Adeninate metal-organic framework for aqueous encapsulation and sensitization of near-infrared and visible emitting lanthanide cations. J. Am. Chem. Soc. 2011, 133, 1220–1223. [Google Scholar] [CrossRef] [PubMed]
- Friebe, L.; Nuyken, O.; Obrecht, W. Neodymium-based Ziegler/Natta catalysts and their application in diene polymerisation. Adv. Polym. Sci. 2006, 204, 1–154. [Google Scholar]
- Kautsky, H. Quenching of luminescence by oxygen. Trans. Faraday Soc. 1939, 35, 216–219. [Google Scholar] [CrossRef]
- Uemura, T.; Hiramatsu, D.; Kubota, Y.; Takata, M.; Kitagawa, S. Topotactic linear radical polymerization of divinylbenzenes in porous coordination polymers. Angew. Chem. Int. Ed. 2007, 46, 4987–4990. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.M.; Guan, Y.F.; Wang, D.Y.; Dong, W.; Wang, X.T.; Gao, S. Syntheses, structures and properties of seven isomorphous 1D Ln3+ complexes Ln(BTA)(HCOO)(H2O)3 (H2BTA = bis(tetrazoly)amine, Ln = Pr, Gd, Eu, Tb, Dy, Er, Yb) and two 3D Ln3+ complexes Ln(HCOO)3 (Ln = Pr, Nd). Dalton Trans. 2008, 6165–6169. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Bonnet, F.; Visseaux, M. Highly efficient cis-1,4 polymerisation of isoprene using simple homoleptic amido rare earth-based catalysts. Polymer 2014, 55, 5013–5016. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russell, S.; Loiseau, T.; Volkringer, C.; Visseaux, M. Luminescent Lanthanide Metal Organic Frameworks for cis-Selective Isoprene Polymerization Catalysis. Inorganics 2015, 3, 467-481. https://doi.org/10.3390/inorganics3040467
Russell S, Loiseau T, Volkringer C, Visseaux M. Luminescent Lanthanide Metal Organic Frameworks for cis-Selective Isoprene Polymerization Catalysis. Inorganics. 2015; 3(4):467-481. https://doi.org/10.3390/inorganics3040467
Chicago/Turabian StyleRussell, Samantha, Thierry Loiseau, Christophe Volkringer, and Marc Visseaux. 2015. "Luminescent Lanthanide Metal Organic Frameworks for cis-Selective Isoprene Polymerization Catalysis" Inorganics 3, no. 4: 467-481. https://doi.org/10.3390/inorganics3040467
APA StyleRussell, S., Loiseau, T., Volkringer, C., & Visseaux, M. (2015). Luminescent Lanthanide Metal Organic Frameworks for cis-Selective Isoprene Polymerization Catalysis. Inorganics, 3(4), 467-481. https://doi.org/10.3390/inorganics3040467