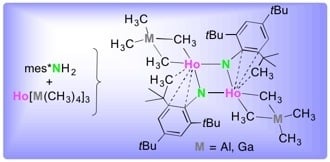
Holmium(III) Supermesityl-Imide Complexes Bearing Methylaluminato/Gallato Ligands
Abstract
:1. Introduction

2. Results and Discussion


| 1 | 2 | ||
|---|---|---|---|
| Ho1–N1′ | 2.107(1) | Ho1–N1′ | 2.102(3) |
| Ho1–N1 | 2.283(1) | Ho1–N1 | 2.288(3) |
| Ho1′–N1 | 2.107(1) | Ho1′–N1 | 2.102(3) |
| Ho1–C1 | 2.512(2) | Ho1–C19 | 2.512(4) |
| Ho1–C2 | 2.598(2) | Ho1–C20 | 2.594(5) |
| Ho1–C5 | 2.626(1) | Ho1–C1 | 2.629(3) |
| Ho1–C6 | 2.715(1) | Ho1–C6 | 2.750(3) |
| Ho1–C13 | 2.833(1) | Ho1–C16 | 2.864(3) |
| Ho1 Al1 | 3.0838(5) | Ho1 Ga1 | 3.0573(4) |
| Al1–C1 | 2.0836(16) | Ga1–C19 | 2.115(4) |
| Al1–C2 | 2.0718(16) | Ga1–C20 | 2.092(4) |
| Al1–C3 | 1.9681(19) | Ga1–C21 | 1.978(4) |
| Al1–C4 | 1.9727(17) | Ga1–C22 | 1.974(4) |
| N1–C5 | 1.3811(18) | N1–C1 | 1.386(4) |
| N1′–Ho1–N1 | 84.63(4) | N1′–Ho1–N1 | 84.68(11) |
| Ho1′–N1–Ho1 | 95.37(4) | Ho1′–N1–Ho1 | 95.32(11) |
| Ho1′–N1–C5 | 175.67(10) | Ho1′–N1–C1 | 176.8(2) |
| Ho1–N1–C5 | 87.95(8) | Ho1–N1–C1 | 87.80(18) |
| C1–Ho1–C2 | 82.76(5) | C19–Ho1–C20 | 83.98(14) |
| C1–Al1–C2 | 108.80(6) | C19–Ga1–C20 | 108.63(15) |
| C3–Al1–C4 | 118.82(8) | C21–Ga1–C22 | 117.5(2) |
| C1–Ho1–C2–Al1 | −11.34(5) | C19–Ho1–C20–Ga1 | 14.20(14) |
| Compounds | Ln–N | CN a | Reference |
|---|---|---|---|
| [(TptBu,Me)Y{NC6H3(CH3)2-2,6}(AlMe3)] c | 2.123(2)–2.128(3) | 5 | [10] |
| [(TptBu,Me)Y{NC6H3(CH3)2-2,6}(HAlMe2)] c | 2.133(2) | 5 | [8] |
| [(TptBu,Me)Y(NtBu)(AlMe3)] c | 2.081(3)–2.088(3) | 5 | [9] |
| [(TptBu,Me)Ho(NtBu)(AlMe3)] c | 2.083(2)–2.084(2) | 5 | [9] |
| [(TptBu,Me)Y(NAd)(AlMe3)] c,d | 2.092(2)–2.099(2) | 5 | [9] |
| [(TptBu,Me)Ho(NAd)(AlMe3)] c,d | 2.087(2)–2.090(2) | 5 | [9] |
| [(TptBu,Me)Y{NC6H3(CH3)2-2,6}(DMAP) c,e | 2.024(4) | 5 | [10] |
| [(C5Me4SiMe3)4Y4(µ3-NCH2CH3)2(µ2-NCHPh)4] | 2.116(6)–2.418(6) | 6/7 | [14] |
| [L3Y3(µ2-CH3)3(µ3-CH3)(µ3-NR)] f | 2.308(3)–2.435(7) | 6 | [28] |
| [Y{Al(CH3)4}(µ2-Nmes*)]2 | 2.1089(9)–2.2909(9) | 6 | [7] |
| [Ho{Al(CH3)4}(µ2-Nmes*)]2 | 2.107(1)–2.283(1) | 6 | b |
| [Ho{Ga(CH3)4}(µ2-Nmes*)]2 | 2.102(3)–2.288(3) | 6 | b |
3. Experimental Section
3.1. General Procedures
3.2. [Ho{M(CH3)4}(µ2-Nmes*)]2 (1 and 2)
3.2.1. Procedure A
3.2.2. Procedure B
3.3. [Ho{Al(CH3)4}(µ2-Nmes*)]2 (1)
3.3.1. Procedure A
3.3.2. Procedure B
3.3.3. Physical Data of 1
3.4. [Ho(GaMe4)(µ2-Nmes*)]2 (2)
3.4.1. Procedure A
3.4.2. Procedure B
3.4.3. Physical Data of 2
3.5. X-Ray Crystallography
| 1 | 2 | |
|---|---|---|
| Formula | C44H82Al2Ho2N2 | C44H82Ga2Ho2N2 |
| Color | Yellow | Yellow |
| Mr (g·mol−1) | 1022.94 | 1108.41 |
| Cryst system | Monoclinic | Triclinic |
| Space group | C2/c | P |
| a [Å] | 24.0888(13) | 10.1896(4) |
| b [Å] | 11.7687(6) | 11.5565(5) |
| c [Å] | 20.4327(11) | 11.5966(5) |
| α [°] | 90 | 65.090(3) |
| β [°] | 111.5590(10) | 83.184(3) |
| γ [°] | 90 | 84.962(4) |
| V [Å3] | 5387.3(5) | 1228.69(9) |
| Z | 4 | 1 |
| F(000) | 2080 | 556 |
| T [K] | 103(2) | 173(2) |
| ρcalcd (g cm3) | 1.261 | 1.498 |
| µ(mm−1) | 2.974 | 4.297 |
| R1 (obsd.) a | 0.0172 | 0.0296 |
| wR2 (all) b | 0.0466 | 0.0661 |
| S c | 1.042 | 1.082 |
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Giesbrecht, G.R.; Gordon, J.C. Lanthanide alkylidene and imido complexes. Dalton Trans. 2004, 2387–2393. [Google Scholar] [CrossRef] [PubMed]
- Summerscales, O.T.; Gordon, J.C. Complexes containing multiple bonding interactions between lanthanoid elements and main-group fragments. RSC Adv. 2013, 3, 6682–6692. [Google Scholar] [CrossRef]
- Trifonov, A.A.; Bochkarev, M.N.; Schumann, H.; Loebel, J. Reduction of azobenzene by naphthaleneytterbium: A tetranuclear ytterbium(III) complex combining 1,2-diphenylhydrazido(2−) and phenylimido ligands. Angew. Chem. Int. Ed. Engl. 1991, 30, 1149–1151. [Google Scholar] [CrossRef]
- Evans, W.J.; Ansari, M.A.; Ziller, J.W.; Khan, S.I. Utility of arylamido ligands in yttrium and lanthanide chemistry. Inorg. Chem. 1996, 35, 5435–5444. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.C.; Giesbrecht, G.R.; Clark, D.L.; Hay, P.J.; Keogh, D.W.; Poli, R.; Scott, B.L.; Watkin, J.G. The first example of a μ2-imido functionality bound to a lanthanide metal center: X-ray crystal structure and DFT study of [(μ-ArN)Sm(μ-NHAr)(μ-Me)AlMe2]2 (Ar = 2,6-iPr2C6H3). Organometallics 2002, 21, 4726–4734. [Google Scholar] [CrossRef]
- Scott, J.; Basuli, F.; Fout, A.R.; Huffmann, J.C.; Mindiola, D.J. Evidence for the existence of a terminal imidoscandium compound: Intermolecular C–H activation and complexation reactions with the transient Sc–NAr species. Angew. Chem. Int. Ed. 2008, 47, 8502–8505. [Google Scholar] [CrossRef] [PubMed]
- Schädle, D.; Schädle, C.; Törnroos, K.W.; Anwander, R. Organoaluminum-assisted formation of rare-earth metal imide complexes. Organometallics 2012, 31, 5101–5107. [Google Scholar] [CrossRef]
- Schädle, C.; Schädle, D.; Eichele, K.; Anwander, R. Methylaluminum-supported rare-earth-metal dihydrides. Angew. Chem. Int. Ed. 2013, 52, 13238–13242. [Google Scholar] [CrossRef] [PubMed]
- Schädle, D.; Maichle-Mössmer, C.; Schädle, C.; Anwander, R. Rare-earth-metal methyl, amide, and imide complexes supported by a superbulky scorpionate ligand. Chem. Eur. J. 2014, 21, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Schädle, D.; Meermann-Zimmermann, M.; Schädle, C.; Maichle-Mössmer, C.; Anwander, R. Rare-earth metal complexes with terminal imido ligands. Eur. J. Inorg. Chem. 2015, 1334–1339. [Google Scholar] [CrossRef]
- Chan, H.S.; Li, H.W.; Xie, Z. Synthesis and structural characterization of imido–lanthanide complexes with a metal–nitrogen multiple bond. Chem. Commun. 2002, 652–653. [Google Scholar] [CrossRef]
- Li, J.; Gao, D.; Hu, H.; Cui, C. Reaction of a bulky amine borane with lanthanide trialkyls. Formation of alkyl lanthanide imide complexes. New J. Chem. 2015, 39, 7567–7570. [Google Scholar] [CrossRef]
- Rad’kov, V.; Dorcet, V.; Carpentier, J.F.; Trifonov, A.; Kirillov, E. Alkylyttrium complexes of amidine–amidopyridinate ligands. Intramolecular C(sp3)–H activation and reactivity studies. Organometallics 2013, 32, 1517–1527. [Google Scholar] [CrossRef]
- Cui, D.; Nishiura, M.; Hou, Z. Lanthanide-imido complexes and their reactions with benzonitrile. Angew. Chem. Int. Ed. 2005, 44, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Wicker, B.F.; Scott, J.; Fout, A.R.; Pink, M.; Mindiola, D.J. Atom-economical route to substituted pyridines via a scandium imide. Organometallics 2011, 30, 2453–2456. [Google Scholar] [CrossRef]
- Lu, E.; Zhou, Q.; Li, Y.; Chu, J.; Chen, Y.; Leng, X.; Sun, J. Reactivity of scandium terminal imido complexes towards metal halides. Chem. Commun. 2012, 48, 3403–3405. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Lu, E.; Chen, Y.; Leng, X. Reversible addition of the Si–H bond of phenylsilane to the Sc=N bond of a scandium terminal imido complex. Organometallics 2012, 32, 1137–1140. [Google Scholar] [CrossRef]
- Schädle, D.; Schädle, C.; Schneider, D.; Maichle-Mössmer, C.; Anwander, R. Versatile Ln2(μ-NR)2-imide platforms for ligand exchange and isoprene polymerization. Organometallics 2015, 34, 4994–5008. [Google Scholar] [CrossRef]
- Chu, J.; Lu, E.; Liu, Z.; Chen, Y.; Leng, X.; Song, H. Reactivity of a scandium terminal imido complex towards unsaturated substrates. Angew. Chem. Int. Ed. 2011, 50, 7677–7680. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Han, X.; Kefalidis, C.E.; Zhou, J.; Maron, L.; Leng, X.; Chen, Y. Lewis acid triggered reactivity of a Lewis base stabilized scandium-terminal imido complex: C–H bond activation, cycloaddition, and dehydrofluorination. J. Am. Chem. Soc. 2014, 136, 10894–10897. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J.; Anwander, R.; Doedens, R.J.; Ziller, J.W. The use of heterometallic bridging moieties to generate tractable lanthanide complexes of small ligands. Angew. Chem. Int. Ed. Engl. 1994, 33, 1641–1644. [Google Scholar] [CrossRef]
- Zimmermann, M.; Frøystein, N.Å.; Fischbach, A.; Sirsch, P.; Dietrich, H.M.; Törnroos, K.W.; Herdtweck, E.; Anwander, R. Homoleptic rare-earth metal (III) tetramethylaluminates: Structural chemistry, reactivity, and performance in isoprene polymerization. Chem. Eur. J. 2007, 13, 8784–8800. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, H.M.; Raudaschl-Sieber, G.; Anwander, R. Trimethylyttrium and trimethyllutetium. Angew. Chem. Int. Ed. 2005, 44, 5303–5306. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, H.M.; Maichle-Mössmer, C.; Anwander, R. Donor-assisted tetramethylaluminate/gallate exchange in organolanthanide complexes: Pushing the limits of Pearson’s HSAB concept. Dalton Trans. 2010, 39, 5783–5785. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, H.M.; Törnroos, K.W.; Herdtweck, E.; Anwander, R. Tetramethylaluminate and tetramethylgallate coordination in rare-earth metal half-sandwich and metallocene complexes. Organometallics 2009, 28, 6739–6749. [Google Scholar] [CrossRef]
- Zimmermann, M.; Takats, J.; Kiel, G.; Törnroos, K.W.; Anwander, R. Ln(III) methyl and methylidene complexes stabilized by a bulky hydrotris(pyrazolyl)borate ligand. Chem. Commun. 2008, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Litlabø, R.; Törnroos, K.W.; Anwander, R. “Metastable” Lu(GaMe4)3 reacts like masked [LuMe3]: Synthesis of an unsolvated lanthanide dimethyl complex. Organometallics 2009, 28, 6646–6649. [Google Scholar] [CrossRef]
- Hong, J.; Zhang, L.; Wang, K.; Zhang, Y.; Weng, L.; Zhou, X. Methylidene rare-earth-metal complex mediated transformations of C=N, N=N and N–H bonds: New routes to imido rare-earth-metal clusters. Chem. Eur. J. 2013, 19, 7865–7873. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J.; Anwander, R.; Ziller, J.W. Inclusion of Al2Me6 in the crystalline lattice of the organometallic complexes LnAl3Me12. Organometallics 1995, 14, 1107–1109. [Google Scholar] [CrossRef]
- Dietrich, H.M.; Meermann, C.; Törnroos, K.W.; Anwander, R. Sounding out the reactivity of trimethylyttrium. Organometallics 2006, 25, 4316–4321. [Google Scholar] [CrossRef]
- Zimmermann, M.; Rauschmaier, D.; Eichele, K.; Törnroos, K.W.; Anwander, R. Amido-stabilized rare-earth metal mixed methyl methylidene complexes. Chem. Commun. 2010, 46, 5346–5348. [Google Scholar] [CrossRef] [PubMed]
- X-Area v. 1.55; Stoe & Cie GmbH: Darmstadt, Germany, 2009.
- X-Red 32 v. 1.53; Stoe & Cie GmbH: Darmstad, Germany, 2009.
- X-Shape v.2.12.2; Stoe & Cie GmbH: Darmstadt, Germany, 2009.
- APEX v. 2012.10_0; Bruker AXS Inc.: Madison, WI, USA, 2012.
- SAINT v. 7.99A; Bruker AXS Inc.: Madison, WI, USA, 2012.
- Sheldrick, G.M. SADABS v. 2012/1; Bruker AXS Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- POV-Ray v. 3.7; Persistence of Vision Pty. Ltd.: Williamstown, Australia, 2004; Available online: http://www.povray.org/ (accessed on 18 March 2014).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schädle, D.; Maichle-Mössmer, C.; Törnroos, K.W.; Anwander, R. Holmium(III) Supermesityl-Imide Complexes Bearing Methylaluminato/Gallato Ligands. Inorganics 2015, 3, 500-510. https://doi.org/10.3390/inorganics3040500
Schädle D, Maichle-Mössmer C, Törnroos KW, Anwander R. Holmium(III) Supermesityl-Imide Complexes Bearing Methylaluminato/Gallato Ligands. Inorganics. 2015; 3(4):500-510. https://doi.org/10.3390/inorganics3040500
Chicago/Turabian StyleSchädle, Dorothea, Cäcilia Maichle-Mössmer, Karl W. Törnroos, and Reiner Anwander. 2015. "Holmium(III) Supermesityl-Imide Complexes Bearing Methylaluminato/Gallato Ligands" Inorganics 3, no. 4: 500-510. https://doi.org/10.3390/inorganics3040500
APA StyleSchädle, D., Maichle-Mössmer, C., Törnroos, K. W., & Anwander, R. (2015). Holmium(III) Supermesityl-Imide Complexes Bearing Methylaluminato/Gallato Ligands. Inorganics, 3(4), 500-510. https://doi.org/10.3390/inorganics3040500