Synthesis and Characterization of Cerium(IV) Metallocenes
Abstract
:1. Introduction
2. Results and Discussion
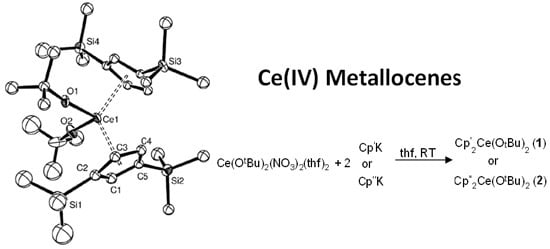
2.1. Preparation of Cerium(IV) Metallocenes


| Ce(1)–O(1) | 2.077(5) | Ce(1)–Cn(1) | 2.508(7) |
| Ce(1)–O(2) | 2.097(6) | Ce(1)–C(12) | 2.798(8) |
| Ce(1)–C(1) | 2.799(9) | Ce(1)–C(13) | 2.791(8) |
| Ce(1)–C(2) | 2.811(9) | Ce(1)–C(14) | 2.764(8) |
| Ce(1)–C(3) | 2.743(9) | Ce(1)–C(15) | 2.774(8) |
| Ce(1)–C(4) | 2.733(9) | Ce(1)–C(16) | 2.812(9) |
| Ce(1)–C(5) | 2.810(8) | Ce(1)–Cn(2) | 2.514(7) |
| O(1)–Ce(1)–Cn(1) | 108.7(4) | O(1)–Ce(1)–Cn(2) | 109.9(8) |
| O(1)–Ce(1)–Cn(2) | 105.8(2) | O(1)–Ce(1)–O(2) | 103.9(2) |
| O(2)–Ce(1)–Cn(1) | 105.6(4) | Cn(1)–Ce(1)–Cn(2) | 121.7(2) |
2.2. Attempted Reactivity of 1 and 2
3. Experimental Section
3.1. Preparation of (Cp′)2Ce(OtBu)2 (1)
3.2. Preparation of (Cp″)2Ce(OtBu)2 (2)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Das, A.K. Kinetic and mechanistic aspects of metal ion catalysis in cerium(IV) oxidation. Coord. Chem. Rev. 2001, 213, 307–325. [Google Scholar] [CrossRef]
- Dziegiec, J.; Domagala, S. The oxidation activity of cerium(IV) ions toward some of the organic compounds. Trends Inorg. Chem. 2005, 8, 43–64. [Google Scholar] [CrossRef]
- Nair, V.; Balagopal, L.; Rajan, R.; Mathew, J. Recent advances in synthetic transformations mediated by cerium(IV) ammonium nitrate. Acc. Chem. Res. 2004, 37, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, M. Sequence-selective scission of DNA and RNA by lanthanide ions and their complexes. Met. Ions Biol. Syst. 2003, 40, 463–475. [Google Scholar] [PubMed]
- Yamamoto, Y.; Komiyama, M. Development of new biotechnology by cerium(IV)-based artificial restriction enzyme. Mater. Integr. 2005, 19, 55–59. [Google Scholar]
- Jian, H.-O.; Zhou, X.-R.; Zhao, D.-F. Recent progress of synthetic methods and applications of cerium β-diketones. Huaxue Shiji 2006, 28, 279–282. [Google Scholar]
- Duprez, D.; Descorme, C. Oxygen storage/redox capacity and related phenomena on ceria-based catalysts. Catal. Sci. Ser. 2002, 2, 243–280. [Google Scholar]
- Imamura, S. Ceria-based wet-oxidation catalysts. Catal. Sci. Ser. 2002, 2, 431–452. [Google Scholar]
- Kaspar, J.; Fornasiero, P. Nanostructured materials for advanced automotive de-pollution catalysts. J. Solid State Chem. 2003, 171, 19–29. [Google Scholar] [CrossRef]
- Kaspar, J.; Fornasiero, P.; Graziani, M. Use of CeO2-based oxides in the three-way catalysis. Catal. Today 1999, 50, 285–298. [Google Scholar] [CrossRef]
- Primet, M.; Garbowski, E. Fundamentals and applications of ceria in combustion reactions. Catal. Sci. Ser. 2002, 2, 407–429. [Google Scholar]
- Shelef, M.; Graham, G.W.; McCabe, R.W. Ceria and other oxygen storage components in automotive catalysts. Catal. Sci. Ser. 2002, 2, 343–375. [Google Scholar]
- Trovarelli, A.; de Leitenburg, C.; Boaro, M.; Dolcetti, G. The utilization of ceria in industrial catalysis. Catal. Today 1999, 50, 353–367. [Google Scholar] [CrossRef]
- Droese, P.; Gottfriedsen, J.; Hrib, C.G.; Jones, P.G.; Hilfert, L.; Edelmann, F.T. The first cationic complex of tetravalent cerium. Z. Anorg. Allg. Chem. 2011, 637, 369–373. [Google Scholar] [CrossRef]
- Aspinall, H.C.; Bacsa, J.; Jones, A.C.; Wrench, J.S.; Black, K.; Chalker, P.R.; King, P.J.; Marshall, P.; Werner, M.; Davies, H.O.; et al. Ce(IV) complexes with donor-functionalized alkoxide ligands: Improved precursors for chemical vapor deposition of CeO2. Inorg. Chem. 2011, 50, 11644–11652. [Google Scholar] [CrossRef] [PubMed]
- Broderick, E.M.; Diaconescu, P.L. Cerium(IV) catalysts for the ring-opening polymerization of lactide. Inorg. Chem. 2009, 48, 4701–4706. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.L.; Casely, I.J.; Zlatogorsky, S.; Wilson, C. Organometallic cerium complexes from tetravalent coordination complexes. Helv. Chim. Acta 2009, 92, 2291–2303. [Google Scholar] [CrossRef]
- Evans, W.J.; Deming, T.J.; Olofson, J.M.; Ziller, J.W. Synthetic and structural studies of a series of soluble cerium(IV) alkoxide and alkoxide nitrate complexes. Inorg. Chem. 1989, 28, 4027–4034. [Google Scholar] [CrossRef]
- Schneider, D.; Spallek, T.; Maichle-Moessmer, C.; Toernroos, K.W.; Anwander, R. Cerium tetrakis(diisopropylamide)—A useful precursor for cerium(IV) chemistry. Chem. Commun. 2014, 50, 14763–14766. [Google Scholar] [CrossRef] [PubMed]
- Eisenstein, O.; Hitchcock, P.B.; Hulkes, A.G.; Lappert, M.F.; Maron, L. Cerium masquerading as a group 4 element: Synthesis, structure and computational characterisation of [CeCl{N(SiMe2)3}]. Chem. Commun. 2001, 1560–1561. [Google Scholar] [CrossRef]
- Hitchcock, P.B.; Hulkes, A.G.; Lappert, M.F. Oxidation in nonclassical organolanthanide chemistry: Synthesis, characterization, and X-ray crystal structures of cerium(III) and -(IV) amides. Inorg. Chem. 2004, 43, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Casely, I.J.; Liddle, S.T.; Blake, A.J.; Wilson, C.; Arnold, P.L. Tetravalent cerium carbene complexes. Chem. Commun. 2007, 5037–5039. [Google Scholar] [CrossRef] [PubMed]
- Werner, D.; Deacon, G.B.; Junk, P.C.; Anwander, R. Cerium(III/IV) formamidinate chemistry, and a stable cerium(IV) diolate. Chemistry 2014, 20, 4426–4438. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; Cesca, S.; Bertolini, W. New 7r-cyclooctate′I′raenyl and iT-cyclopentadienyl complexes of cerium. J. Organomet. Chem. 1976, 113, 321–330. [Google Scholar] [CrossRef]
- Gulino, A.; Casarin, M.; Conticello, V.P.; Gaudiello, J.G.; Mauermann, H.; Fragala, I.; Marks, T.J. Efficient synthesis, redox characteristics, and electronic structure of a tetravalent tris(cyclopentadienyl)cerium alkoxide complex. Organometallics 1988, 7, 2360–2364. [Google Scholar] [CrossRef]
- Kalsotra, B.L.; Multani, R.K.; Jain, B.D. Preparation and properties of tricyclopentadienyl cerium(IV) chloride and bisindenyl cerium(IV) dichloride. Isr. J. Chem. 1971, 9, 569–572. [Google Scholar] [CrossRef]
- Deacon, G.B.; Tuong, T.D.; Vince, D.G. Refutation of the synthesis of tetrakis(cyclopentadienyl)cerium(IV). Polyhedron 1983, 2, 969–970. [Google Scholar] [CrossRef]
- Evans, W.J.; Deming, T.J.; Ziller, J.W. The utility of ceric ammonium nitrate-derived alkoxide complexes in the synthesis of organometallic cerium(IV) complexes. Synthesis and first X-ray crystallographic detrmination of a tetravalent cerium cyclopentadienide complex, (C5H5)3Ce(OCMe3). Organometallics 1989, 8, 1581–1583. [Google Scholar] [CrossRef]
- Dröse, P.; Crozier, A.R.; Lashkari, S.; Gottfriedsen, J.; Blaurock, S.; Hrib, C.G.; Maichle-Mössmer, C.; Schädle, C.; Anwander, R.; Edelmann, F.T. Facile access to tetravalent cerium compounds: One-electron oxidation using iodine(III) reagents. J. Am. Chem. Soc. 2010, 132, 14046–14047. [Google Scholar] [CrossRef] [PubMed]
- APEX II 1.08, Bruker AXS, Inc.: Madison, WI, USA, 2004.
- SAINT+ 7.06, Bruker AXS, Inc.: Madison, WI, USA, 2003.
- Sheldrick, G. SADABS 2.03, University of Göttingen: Göttingen, Germany, 2001.
- SHELXTL 5.10, Bruker AXS, Inc.: Madison, WI, USA, 1997.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutton, A.D.; Clark, D.L.; Scott, B.L.; Gordon, J.C. Synthesis and Characterization of Cerium(IV) Metallocenes. Inorganics 2015, 3, 589-596. https://doi.org/10.3390/inorganics3040589
Sutton AD, Clark DL, Scott BL, Gordon JC. Synthesis and Characterization of Cerium(IV) Metallocenes. Inorganics. 2015; 3(4):589-596. https://doi.org/10.3390/inorganics3040589
Chicago/Turabian StyleSutton, Andrew D., David L. Clark, Brian L. Scott, and John C. Gordon. 2015. "Synthesis and Characterization of Cerium(IV) Metallocenes" Inorganics 3, no. 4: 589-596. https://doi.org/10.3390/inorganics3040589
APA StyleSutton, A. D., Clark, D. L., Scott, B. L., & Gordon, J. C. (2015). Synthesis and Characterization of Cerium(IV) Metallocenes. Inorganics, 3(4), 589-596. https://doi.org/10.3390/inorganics3040589