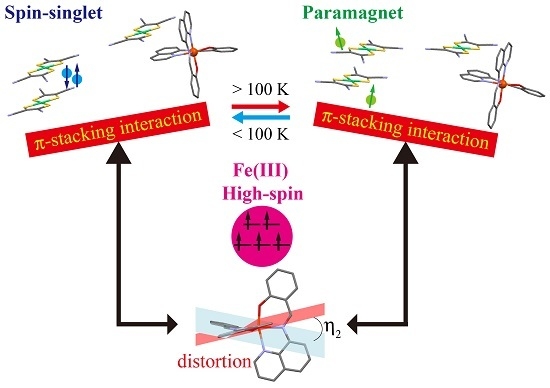
Spin-Singlet Transition in the Magnetic Hybrid Compound from a Spin-Crossover Fe(III) Cation and π-Radical Anion
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Magnetic Susceptibility
2.3. Crystal Structural Analysis
2.3.1. Molecular Structures in 1 at 293 K
2.3.2. Molecular Arrangement of 1 at 293 K
2.3.3. Thermal Variations of the Crystal Structure of 1
2.4. Transfer Integrals Between the [Ni(mnt)2] Molecules
2.5. Electron Paramagnetic Resonance (EPR) Spectra
2.6. Mössbauer Spectra
3. Materials and Methods
3.1. Synthesis of [Fe(qsal)2][Ni(mnt)2] (1)
3.2. Physical Measurements
3.3. Crystal Structure Determinations
3.4. Transfer Integral Calculations
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gütlich, P. Spin Crossover in Transition Metal Compounds; Goodwin, H.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Halcrow, M.A. Spin-Crossover Materials; John Wiley & Sons, Ltd.: Oxford, UK, 2013. [Google Scholar]
- Bousseksou, A.; Molnár, G.; Salmon, L.; Nicolazzi, W. Molecular spin crossover phenomenon: Recent achievements and prospects. Chem. Soc. Rev. 2011, 40, 3313–3335. [Google Scholar] [CrossRef] [PubMed]
- Gütlich, P.; Gaspar, A.B.; Garcia, Y. Spin state switching in iron coordination compounds. Beilstein J. Org. Chem. 2013, 9, 342–391. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Cui, H.-B.; Okano, Y.; Kobayashi, H.; Einaga, Y.; Sato, O. Electrical conductivity modulation coupled to a high-spin–low-spin conversion in the molecular system [FeIII(qsal)2][Ni(dmit)2]3·CH3CN·H2O. Inorg. Chem. 2006, 45, 5739–5741. [Google Scholar] [CrossRef] [PubMed]
- Faulmann, C.; Jacob, K.; Dorbes, S.; Lampert, S.; Malfant, I.; Doublet, M.-L.; Valade, L.; Real, J.A. Electrical conductivity and spin crossover: A new achievement with a metal bis dithiolene complex. Inorg. Chem. 2007, 46, 8548–8559. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Cui, H.-B.; Okano, Y.; Kobayashi, H.; Mori, H.; Tajima, H.; Einaga, Y.; Sato, O. Evidence of the chemical uniaxial strain effect on electrical conductivity in the spin-crossover conducting molecular system: [FeIII(qnal)2][Pd(dmit)2]5·acetone. J. Am. Chem. Soc. 2008, 130, 6688–6689. [Google Scholar] [CrossRef] [PubMed]
- Djukic, B.; Lemaire, M.T. Hybrid Spin-crossover conductor exhibiting unusual variable-temperature electrical conductivity. Inorg. Chem. 2009, 48, 10489–10491. [Google Scholar] [CrossRef] [PubMed]
- Nihei, M.; Takahashi, N.; Nishikawa, H.; Oshio, H. Spin-crossover behavior and electrical conduction property in iron(II) complexes with tetrathiafulvalene moieties. Dalton Trans. 2011, 40, 2154–2156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, H.; Benjamin, S.M.; Steven, E.; Brooks, J.S.; Shatruk, M. Photomagnetic response in highly conductive iron(II) spin-crossover complexes with TCNQ radicals. Angew. Chem. Int. Ed. 2015, 54, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Z.-X.; Xie, H.; Li, M.-X.; Woods, T.J.; Dunbar, K.R. A cobalt(II) spin-crossover compound with partially charged TCNQ radicals and an anomalous conducting behavior. Chem. Sci. 2016, 7, 1569–1574. [Google Scholar] [CrossRef]
- Shvachko, Y.N.; Starichenko, D.V.; Korolyov, A.V.; Yagubskii, E.B.; Kotov, A.I.; Buravov, L.I.; Lyssenko, K.A.; Zverev, V.N.; Simonov, S.V.; Zorina, L.V.; et al. The conducting spin-crossover compound combining Fe(II) cation complex with TCNQ in a fractional reduction state. Inorg. Chem. 2016, 55, 9121–9130. [Google Scholar] [CrossRef] [PubMed]
- Clemente-León, M.; Coronado, E.; López-Jordà, M.; Soriano-Portillo, A.; Waerenborgh, J.C.; Delgado, F.S.; Ruiz-Pérez, C. Insertion of a spin crossover FeIII complex into an oxalate-based layered material: Coexistence of spin canting and spin crossover in a hybrid magnet. Inorg. Chem. 2008, 47, 9111–9120. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.I.S.; Dias, J.C.; Vieira, B.J.C.; Santos, I.C.; Branco, M.B.C.; Pereira, L.C.J.; Waerenborgh, J.C.; Almeida, M.; Belo, D.; Gama, V. A new hybrid material exhibiting room temperature spin-crossover and ferromagnetic cluster-glass behavior. CrystEngComm 2009, 11, 2160–2168. [Google Scholar] [CrossRef]
- Nihei, M.; Tahira, H.; Takahashi, N.; Otake, Y.; Yamamura, Y.; Saito, K.; Oshio, H. Multiple bistability and tristability with dual spin-state conversions in [Fe(dpp)2][Ni(mnt)2]2·MeNO2. J. Am. Chem. Soc. 2010, 132, 3553–3560. [Google Scholar] [CrossRef] [PubMed]
- Clemente-León, M.; Coronado, E.; López-Jordà, M.; Espallargas, G.M.; Soriano-Portillo, A.; Waerenborgh, J.C. Multifunctional magnetic materials obtained by insertion of a spin-crossover FeIII complex into bimetallic oxalate-based ferromagnets. Chem. Eur. J. 2010, 16, 2207–2219. [Google Scholar] [CrossRef] [PubMed]
- Ohkoshi, S.; Imoto, K.; Tsunobuchi, Y.; Takano, S.; Tokoro, H. Light-induced spin-crossover magnet. Nat. Chem. 2011, 3, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Clemente-León, M.; Coronado, E.; López-Jordà, M.; Waerenborgh, J.C. Multifunctional magnetic materials obtained by insertion of spin-crossover FeIII complexes into chiral 3D bimetallic oxalate-based ferromagnets. Inorg. Chem. 2011, 50, 9122–9130. [Google Scholar] [CrossRef] [PubMed]
- Roubeau, O.; Evangelisti, M.; Natividad, E. A spin crossover ferrous complex with ordered magnetic ferric anions. Chem. Commun. 2012, 48, 7604–7606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemente-León, M.; Coronado, E.; López-Jordà, M.; Waerenborgh, J.C.; Desplanches, C.; Wang, H.; Létard, J.-F.; Hauser, A.; Tissot, A. Stimuli responsive hybrid magnets: Tuning the photoinduced spin-crossover in Fe(III) complexes inserted into layered magnets. J. Am. Chem. Soc. 2013, 135, 8655–8667. [Google Scholar] [CrossRef] [PubMed]
- Ababei, R.; Pichon, C.; Roubeau, O.; Li, Y.-G.; Bréfuel, N.; Buisson, L.; Guionneau, P.; Mathonière, C.; Clérac, R. Rational design of a photomagnetic chain: Bridging single-molecule magnets with a spin-crossover complex. J. Am. Chem. Soc. 2013, 135, 14840–14853. [Google Scholar] [CrossRef] [PubMed]
- Fukuroi, K.; Takahashi, K.; Mochida, T.; Sakurai, T.; Ohta, H.; Yamamoto, T.; Einaga, Y.; Mori, H. Synergistic spin transition between spin crossover and spin-Peierls-like singlet formation in the halogen-bonded molecular hybrid system: [Fe(Iqsal)2][Ni(dmit)2]·CH3CN·H2O. Angew. Chem. Int. Ed. 2014, 53, 1983–1986. [Google Scholar] [CrossRef] [PubMed]
- Okai, M.; Takahashi, K.; Sakurai, T.; Ohta, H.; Yamamoto, T.; Einaga, Y. Novel Fe(II) spin crossover complexes involving a chalcogen-bond and π-stacking interactions with a paramagnetic and nonmagnetic M(dmit)2 anion (M=Ni, Au; dmit = 4,5-dithiolato-1,3-dithiole-2-thione). J. Mater. Chem. C 2015, 3, 7858–7864. [Google Scholar] [CrossRef]
- Ohkoshi, S.; Takano, S.; Imoto, K.; Yoshikiyo, M.; Namai, A.; Tokoro, H. 90-degree optical switching of output second-harmonic light in chiral photomagnet. Nat. Photonics 2014, 8, 65–71. [Google Scholar] [CrossRef]
- Wang, C.F.; Li, R.-F.; Chen, X.-Y.; Wei, R.-J.; Zheng, L.-S.; Tao, J. Synergetic spin crossover and fluorescence in one-dimensional hybrid complexes. Angew. Chem. Int. Ed. 2015, 54, 1574–1577. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Cui, H.-B.; Kobayashi, H.; Einaga, Y.; Sato, O. The light-induced excited spin state trapping effect on Ni(dmit)2 salt with an Fe(III) spin-crossover cation: [Fe(qsal)2][Ni(dmit)2]·2CH3CN. Chem. Lett. 2005, 34, 1240–1241. [Google Scholar] [CrossRef]
- Takahashi, K.; Mori, H.; Kobayashi, H.; Sato, O. Mechanism of reversible spin transition with a thermal hysteresis loop in [FeIII(qsal)2][Ni(dmise)2]·2CH3CN: Selenium analogue of the precursor of an Fe(III) spin-crossover molecular conducting system. Polyhedron 2009, 28, 1776–1781. [Google Scholar] [CrossRef]
- Duana, H.-B.; Ren, X.-M.; Meng, Q.-J. One-dimensional (1D) [Ni(mnt)2]–-based spin-Peierls-like complexes: Structural, magnetic and transition properties. Coord. Chem. Rev. 2010, 254, 1509–1522. [Google Scholar] [CrossRef]
- Dickinson, R.C.; Baker, W.A.; Collins, R.L. The magnetic properties of bis[N-(8-quinolyl)-salicylaldimine]halogenoiron(III)·X hydrate, Fe(8-QS)2X·xH2O: A reexamination. J. Inorg. Nucl. Chem. 1977, 39, 1531–1533. [Google Scholar] [CrossRef]
- Kobayashi, A.; Sasaki, Y. One-dimensional system of square-planar bis(1,2-dicyanovinylene-1,2-dithiolato) metal complexes. I. The crystal structure of [(C4H9)4N]2[Ni(mnt)2] and [(C2H5)4N][Ni(mnt)2]. Bull. Chem. Soc. Jpn. 1977, 50, 2650–2656. [Google Scholar] [CrossRef]
- Mori, T.; Kobayashi, A.; Sasaki, Y.; Kobayashi, H.; Saito, G.; Inokuchi, H. The intermolecular interaction of tetrathiafulvarene in organic metals. Calculation of orbital overlaps and models of energy-band structures. Bull. Chem. Soc. Jpn. 1984, 57, 627–633. [Google Scholar] [CrossRef]
- Akutagawa, T.; Nakamura, T.; Inabe, T.; Underhill, A.E. Structures of Ni(dmit)2 salts of lithium or ammonium included in crown ether assemblies. Thin Solid Films 1998, 331, 264–271. [Google Scholar] [CrossRef]
- Akutagawa, T.; Nakamura, T. Control of assembly and magnetism of metal-dmit complexes by supramolecular cations. Coord. Chem. Rev. 2002, 226, 3–9. [Google Scholar] [CrossRef]
- Ivanova, T.A.; Ovchinnikov, I.V.; Garipov, R.R.; Ivanova, G.I. Spin crossover [Fe(qsal)2]X (X = Cl, SCN, CF3SO3) complexes: EPR and DFT study. Appl. Magn. Reson. 2011, 40, 1–10. [Google Scholar] [CrossRef]
- Zhou, H.; Wen, L.L.; Ren, X.M.; Meng, Q.J. Novel molecular staircases constructing from H–bonding interactions based on the building blocks of [Ni(mnt)2]– ions: Syntheses, crystal structures, EPR spectra and magnetic properties. J. Mol. Struct. 2006, 787, 31–37. [Google Scholar] [CrossRef]
- Hijmans, T.W.; Beyermann, W.P. Electron-spin-resonance study of the high-field phase of the spin-Peierls system tetrathiafulvalene-Au-bis-dithiolene. Phys. Rev. Lett. 1987, 58, 2351–2354. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Ohta, H.; Motokawa, M.; Fujita, O.; Akimitsu, J. The observation of g-shifts in spin-Peierls material CuGeO3 by submillimeter wave ESR. J. Phys. Soc. Jpn. 1997, 66, 1115–1123. [Google Scholar] [CrossRef]
- Oshio, H.; Kitazaki, K.; Mishiro, J.; Kato, N.; Maeda, Y.; Takashima, Y. New spin-crossover iron(III) complexes with large hysteresis effects and time dependence of their magnetism. J. Chem. Soc. Dalton Trans. 1987, 1341–1347. [Google Scholar] [CrossRef]
- Hayami, S.; Gu, Z.-Z.; Yoshiki, H.; Fujishima, A.; Sato, O. Iron(III) spin-crossover compounds with a wide apparent thermal hysteresis around room temperature. J. Am. Chem. Soc. 2001, 123, 11644–11650. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Mori, H.; Tajima, H.; Einaga, Y.; Sato, O. Cooperative spin transition and thermally quenched high-spin state in new polymorph of [Fe(qsal)2]I3. Hyperfine Interact. 2012, 206, 1–5. [Google Scholar] [CrossRef]
- Bousseksou, A.; Place, C.; Linares, J.; Varret, F. Dynamic spin crossover in [Fe(2-BIK)3](ClO4)2 and [Fe(Me22-BIK)3](BF4)2 investigated by Mossbauer spectroscopy. J. Mag. Mag. Mater. 1992, 104–107, 225–226. [Google Scholar] [CrossRef]
- Maeda, Y.; Tsutsumi, N.; Takashima, Y. Examples of fast and slow electronic relaxation between 6A and 2T. Inorg. Chem. 1984, 23, 2440–2447. [Google Scholar] [CrossRef]
- Wignall, J.W.G. Mössbauer line broadening in trivalent iron compounds. J. Chem. Phys. 1966, 44, 2462–2467. [Google Scholar] [CrossRef]
- Summerville, R.H.; Hoffmann, R. Tetrahedral and other M2L6 transition metal dimers. J. Am. Chem. Soc. 1976, 98, 7240–7254. [Google Scholar] [CrossRef]
- Chen, M.M.L.; Hoffmann, R. Sulfuranes. Theoretical aspects of bonding, substituent site preferences, and geometrical distortions. J. Am. Chem. Soc. 1976, 98, 1647–1653. [Google Scholar] [CrossRef]
- Jørgensen, K.A.; Hoffmann, R. Binding of alkenes to the ligands in OsO2X2 (X = O and NR) and CpCo(NO)2. A frontier orbital study of the formation of intermediates in the transition-metal-catalyzed synthesis of diols, amino alcohols, and diamines. J. Am. Chem. Soc. 1986, 108, 1867–1876. [Google Scholar] [CrossRef]
- Murata, S.; Takahashi, K.; Sakurai, T.; Ohta, H.; Yamamoto, T.; Einaga, Y.; Shiota, Y.; Yoshizawa, K. The role of Coulomb interactions for spin crossover behaviors and crystal structural transformation in novel anionic Fe(III) complexes from a π-extended ONO ligand. Crystals 2016, 6, 49. [Google Scholar] [CrossRef]
- Murata, S.; Takahashi, K.; Sakurai, T.; Ohta, H. Single-crystal-to-single-crystal transformation in hydrogen-bond-induced high-spin pseudopolymorphs from protonated cation salts with a π-extended spin crossover Fe(III) complex anion. Polyhedron 2017. [Google Scholar] [CrossRef]






| 1 | |||
|---|---|---|---|
| Formula | C40H22FeN8NiO2S4 | ||
| Formula Weight | 889.45 | ||
| Color | black | ||
| Dimension/mm | 0.20 × 0.20 × 0.05 | ||
| T/K | 293 | 100 | 25 |
| Crystal system | triclinic | triclinic | triclinic |
| Space Group | |||
| a/Å | 11.7638(6) | 11.5777(7) | 11.4218(11) |
| b/Å | 13.5893(5) | 13.6154(4) | 13.7207(6) |
| c/Å | 14.1000(10) | 13.9687(11) | 13.8041(17) |
| α/° | 65.962(11) | 66.134(8) | 67.123(12) |
| β/° | 85.184(14) | 84.758(12) | 84.938(18) |
| γ/° | 65.815(9) | 65.272(9) | 65.324(14) |
| V/Å3 | 1868.5(3) | 1821.0(3) | 1804.0(4) |
| Z | 2 | 2 | 2 |
| ρcalcd./g·cm−3 | 1.581 | 1.622 | 1.637 |
| μ (Mo-Kα) | 1.165 | 1.195 | 1.206 |
| 2θmax/° | 55.13 | 54.99 | 55.02 |
| No. Reflections (Rint) | 20124 | 17468 | 17099 |
| (0.0214) | (0.0365) | (0.0347) | |
| No. Observations (I > 2.00 σ(I)) | 8382 | 7972 | 7888 |
| (6421) | (6636) | (6604) | |
| No. Variables | 505 | 505 | 505 |
| R1 (I > 2.00 σ(I)) | 0.0468 | 0.0481 | 0.0553 |
| R (all data) | 0.0600 | 0.0567 | 0.0646 |
| wR2 (all data) | 0.1427 | 0.1309 | 0.1231 |
| Residual electron density/eÅ−3 | 0.90 | 1.14 | 1.05 |
| −0.66 | −0.93 | −1.22 | |
| Goodness of fit | 1.062 | 1.115 | 1.076 |
| 1 | HS [Fe(qsal)2]+ | LS [Fe(qsal)2]+ | |||
|---|---|---|---|---|---|
| T/K | 293 | 100 | 25 | 273 | 200 |
| Fe1–O1/Å | 1.9278(19) | 1.9371(18) | 1.942(2) | 1.918(2) | 1.8806(19) |
| Fe1–O2/Å | 1.908(2) | 1.9131(18) | 1.914(2) | 1.909(3) | 1.882(2) |
| Fe1–N1/Å | 2.097(2) | 2.101(2) | 2.100(3) | 2.114(3) | 1.950(3) |
| Fe1–N2/Å | 2.195(2) | 2.196(2) | 2.195(3) | 2.151(2) | 1.979(2) |
| Fe1–N3/Å | 2.131(2) | 2.132(2) | 2.132(3) | 2.126(3) | 1.948(3) |
| Fe1–N4/Å | 2.136(2) | 2.129(2) | 2.128(3) | 2.133(3) | 1.972(3) |
| Σ 1/° | 83.1(3) | 85.7(3) | 86.1 (4) | 71.5(4) | 44.6(4) |
| Θ 2/° | 149.1(4) | 151.0(4) | 151.6(5) | 121.1(5) | 54.9(5) |
| φ 3/° | 162.17(8) | 161.97(8) | 162.16(11) | 166.35(11) | 176.59(11) |
| η1 4/° | 1.37 | 1.79 | 2.26 | 7.49 | 6.76 |
| η2 5/° | 24.76 | 26.66 | 27.46 | 7.94 | 5.88 |
| this work | this work | this work | Ref. [27] | Ref. [27] | |
| 1 | [Ni(mnt)2]− | [Ni(mnt)2]2− | |||
|---|---|---|---|---|---|
| T/K | 293 | 100 | 25 | ||
| Ni1–S1/Å | 2.1466(8) | 2.1509(7) | 2.1466(10) | 2.147(3) 1 | 2.176(1) 1 |
| Ni1–S2/Å | 2.1383(8) | 2.1432(7) | 2.1459(10) | 2.151(3) 1 | 2.173(1) 1 |
| Ni1–S3/Å | 2.1340(8) | 2.1391(7) | 2.1376(10) | 2.148(3) 1 | − |
| Ni1–S4/Å | 2.1421(8) | 2.1469(7) | 2.1458(10) | 2.149(3) 1 | − |
| S1–C35/Å | 1.718(3) | 1.718(3) | 1.708(4) | 1.727(10) 1 | 1.732(4) 1 |
| S2–C36/Å | 1.715(3) | 1.721(3) | 1.712(4) | 1.722(9) 1 | 1.725(5) 1 |
| S3–C37/Å | 1.715(3) | 1.723(2) | 1.722(4) | 1.705(10) 1 | − |
| S4–C38/Å | 1.720(3) | 1.726(3) | 1.729(4) | 1.724(9) 1 | − |
| C35–C36/Å | 1.349(4) | 1.360(4) | 1.374(5) | 1.367(12) 1 | 1.360(7) 1 |
| C37–C38/Å | 1.358(4) | 1.361(4) | 1.358(5) | 1.370(12) 1 | − |
| this work | this work | this work | Ref. [30] | Ref. [30] | |
| Position 1 | 1 | |||
|---|---|---|---|---|
| T/K | 293 | 100 | 25 | |
| Fe(qsal)2 intradimer | p | |||
| π-plane (qsal···qsal) | 3.405 | 3.346 | 3.331 | |
| Fe1···Fe1 | 6.672 | 6.640 | 6.671 | |
| Fe(qsal)2 interdimer | q, r | |||
| π-plane (quinolyl···quinolyl) | q | 3.347 | 3.297 | 3.302 |
| Fe1···Fe1 | 8.904 | 8.837 | 8.791 | |
| π-plane (phenyl···phenyl) | r | 3.501 | 3.388 | 3.372 |
| Fe1···Fe1 | 9.972 | 9.962 | 10.038 | |
| Ni(mnt)2 intradimer | u | |||
| π-plane | 3.505 | 3.450 | 3.400 | |
| Ni1···Ni1 | 4.028 | 4.002 | 4.142 | |
| Ni(mnt)2 interdimer | v | |||
| π-plane | 3.196 | 3.106 | 3.072 | |
| Ni1···Ni1 | 8.578 | 8.394 | 7.945 |
| 1 | [Ni(dmise)2] | ||||
|---|---|---|---|---|---|
| T/K | 293 | 100 | 25 | 273 | 200 |
| Quinolyl···Ni1 | 3.348 | 3.309 | 3.297 | 3.419 | 3.325 |
| Quinolyl···S1 | 3.288 | 3.244 | 3.301 | 3.579 2 | 3.519 2 |
| Quinolyl···C35 | 3.402 | 3.348 | 3.367 | 3.494 2 | 3.498 2 |
| Quinolyl···C36 | 3.448 | 3.388 | 3.372 | 3.365 2 | 3.404 2 |
| Quinolyl···S2 | 3.448 | 3.393 | 3.362 | 3.297 2 | 3.278 2 |
| Fe1···Ni1 | 6.214 | 6.223 | 6.433 | 6.591 | 6.282 |
| this work | this work | this work | Ref. [27] | Ref. [27] | |
| Position 1 | 1 | |||
|---|---|---|---|---|
| T/K | 293 | 100 | 25 | |
| Intradimer | u | 51.53 | 66.62 | 172.0 |
| Interdimer | v | 22.91 | 28.49 | 25.17 |
| T/K | Spin-State | Ratio | IS 1/mm·s−1 | QS 2/mm·s−1 | LW 3/mm·s−1 |
|---|---|---|---|---|---|
| 293 | HS | 100% | 0.307(16) | 0.91(2) | 0.79(4) |
| 100 | HS | 100% | 0.48(3) | 1.07(3) | 1.54(5) |
| 40 | HS | 100% | 0.65(4) | 1.18(6) | 1.73(5) |
| 15 | HS | 100% | 0.65(3) | 1.24(4) | 1.73(4) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, K.; Sakurai, T.; Zhang, W.-M.; Okubo, S.; Ohta, H.; Yamamoto, T.; Einaga, Y.; Mori, H. Spin-Singlet Transition in the Magnetic Hybrid Compound from a Spin-Crossover Fe(III) Cation and π-Radical Anion. Inorganics 2017, 5, 54. https://doi.org/10.3390/inorganics5030054
Takahashi K, Sakurai T, Zhang W-M, Okubo S, Ohta H, Yamamoto T, Einaga Y, Mori H. Spin-Singlet Transition in the Magnetic Hybrid Compound from a Spin-Crossover Fe(III) Cation and π-Radical Anion. Inorganics. 2017; 5(3):54. https://doi.org/10.3390/inorganics5030054
Chicago/Turabian StyleTakahashi, Kazuyuki, Takahiro Sakurai, Wei-Min Zhang, Susumu Okubo, Hitoshi Ohta, Takashi Yamamoto, Yasuaki Einaga, and Hatsumi Mori. 2017. "Spin-Singlet Transition in the Magnetic Hybrid Compound from a Spin-Crossover Fe(III) Cation and π-Radical Anion" Inorganics 5, no. 3: 54. https://doi.org/10.3390/inorganics5030054
APA StyleTakahashi, K., Sakurai, T., Zhang, W. -M., Okubo, S., Ohta, H., Yamamoto, T., Einaga, Y., & Mori, H. (2017). Spin-Singlet Transition in the Magnetic Hybrid Compound from a Spin-Crossover Fe(III) Cation and π-Radical Anion. Inorganics, 5(3), 54. https://doi.org/10.3390/inorganics5030054