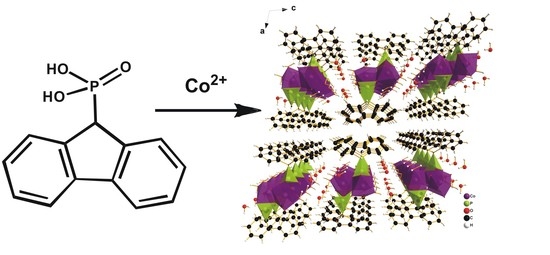
One-Dimensional Fluorene-Based Co(II) Phosphonate Co(H2O)2PO3C–C12H9·H2O: Structure and Magnetism
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Study
2.2. Magnetic Properties
3. Experimental Section
3.1. Synthesis
3.2. Thermogravimetric Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clearfield, A.; Demadis, K. Metal Phosphonate Chemistry: From Synthesis to Applications; Royal Society of Chemistry: Cambridge, UK, 2011; ISBN 978-1-84973-356-4. [Google Scholar]
- Silbernagel, R.; Martin, C.H.; Clearfield, A. Zirconium(IV) Phosphonate-Phosphates as Efficient Ion-Exchange Materials. Inorg. Chem. 2016, 55, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhou, B.; Zhou, H.; Wu, L.; Meng, Q.; Liu, Z.; Han, B. Porous Zirconium–Phytic Acid Hybrid: A Highly Efficient Catalyst for Meerwein–Ponndorf–Verley Reductions. Angew. Chem. Int. Ed. 2015, 54, 9399–9403. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Yee, G.T.; Lin, W. Magnetically Recoverable Chiral Catalysts Immobilized on Magnetite Nanoparticles for Asymmetric Hydrogenation of Aromatic Ketones. J. Am. Chem. Soc. 2005, 127, 12486–12487. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Heising, J.M.; Clearfield, A. Sulfonated Microporous Organic−Inorganic Hybrids as Strong Bronsted Acids1. J. Am. Chem. Soc. 2003, 125, 10375–10383. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Ngo, H.L.; Lin, W. Chiral Porous Hybrid Solids for Practical Heterogeneous Asymmetric Hydrogenation of Aromatic Ketones. J. Am. Chem. Soc. 2003, 125, 11490–11491. [Google Scholar] [CrossRef] [PubMed]
- Maillet, C.; Janvier, P.; Pipelier, M.; Praveen, T.; Andres, Y.; Bujoli, B. Hybrid Materials for Catalysis? Design of New Phosphonate-Based Supported Catalysts for the Hydrogenation of Ketones under Hydrogen Pressure. Chem. Mater. 2001, 13, 2879–2884. [Google Scholar] [CrossRef]
- Mao, J.-G. Structures and luminescent properties of lanthanide phosphonates. Coord. Chem. Rev. 2007, 251, 1493–1520. [Google Scholar] [CrossRef]
- Rueff, J.-M.; Barrier, N.; Boudin, S.; Dorcet, V.; Caignaert, V.; Boullay, P.; Hix, G.B.; Jaffrès, P.-A. Remarkable thermal stability of Eu(4-phosphonobenzoate): Structure investigations and luminescence properties. Dalton Trans. 2009, 10614–10620. [Google Scholar] [CrossRef] [PubMed]
- Mutelet, B.; Boudin, S.; Pérez, O.; Rueff, J.M.; Labbé, C.; Jaffrès, P.A. La1−xLnxH(O3PCH3)2 (Ln = Tb, Eu; 0 < x ≤ 1): An organic-inorganic hybrid with lanthanide chains and tunable luminescence properties. Dalton Trans. 2015, 44, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Colodrero, R.M.P.; Olivera-Pastor, P.; Losilla, E.R.; Aranda, M.A.G.; Leon-Reina, L.; Papadaki, M.; McKinlay, A.C.; Morris, R.E.; Demadis, K.D.; Cabeza, A. Multifunctional lanthanum tetraphosphonates: Flexible, ultramicroporous and proton-conducting hybrid frameworks. Dalton Trans. 2012, 41, 4045–4051. [Google Scholar] [CrossRef] [PubMed]
- Horike, S.; Umeyama, D.; Kitagawa, S. Ion Conductivity and Transport by Porous Coordination Polymers and Metal–Organic Frameworks. Acc. Chem. Res. 2013, 46, 2376–2384. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Dawson, K.W.; Gelfand, B.S.; Taylor, J.M.; Shimizu, G.K.H. Enhancing Proton Conduction in a Metal–Organic Framework by Isomorphous Ligand Replacement. J. Am. Chem. Soc. 2013, 135, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Donnadio, A.; Nocchetti, M.; Costantino, F.; Taddei, M.; Casciola, M.; da Silva Lisboa, F.; Vivani, R. A layered mixed zirconium phosphate/phosphonate with exposed carboxylic and phosphonic groups: X-ray powder structure and proton conductivity properties. Inorg. Chem. 2014, 53, 13220–13226. [Google Scholar] [CrossRef] [PubMed]
- Berchel, M.; Gall, T.L.; Denis, C.; Hir, S.L.; Quentel, F.; Elléouet, C.; Montier, T.; Rueff, J.-M.; Salaün, J.-Y.; Haelters, J.-P.; et al. A silver-based metal–organic framework material as a ‘reservoir’ of bactericidal metal ions. New J. Chem. 2011, 35, 1000–1003. [Google Scholar] [CrossRef]
- Rueff, J.-M.; Perez, O.; Caignaert, V.; Hix, G.; Berchel, M.; Quentel, F.; Jaffrès, P.-A. Silver-Based Hybrid Materials from meta- or para-Phosphonobenzoic Acid: Influence of the Topology on Silver Release in Water. Inorg. Chem. 2015, 54, 2152–2159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, S.-S.; Zheng, L.-M. Magnetic materials based on 3d metal phosphonates. Coord. Chem. Rev. 2016, 319, 63–85. [Google Scholar] [CrossRef]
- Zheng, L.-M.; Duan, Y. Structural and Magnetic Studies of Cobalt Phosphonates. In Metal Phosphonate Chemistry; Clearfield, A., Demadis, K., Eds.; Royal Society of Chemistry: Cambridge, UK, 2011; Chapter 8; pp. 235–278. ISBN 978-1-84973-356-4. [Google Scholar]
- Yin, P.; Gao, S.; Zheng, L.-M. Xin Magnetic Properties of Metal Diphosphonate Compounds with One-Dimensional Chain Structures. Chem. Mater. 2003, 15, 3233–3236. [Google Scholar] [CrossRef]
- Zheng, L.-M.; Gao, S.; Yin, P. Xin One-Dimensional Cobalt Diphosphonates Exhibiting Weak Ferromagnetism and Field-Induced Magnetic Transitions. Inorg. Chem. 2004, 43, 2151–2156. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Gao, S.; Wang, Z.-M.; Yan, C.-H.; Zheng, L.-M. Xin Field-Induced Magnetic Transitions in Metal Phosphonates with Ladderlike Chain Structures: (NH3C6H4NH3)M2(hedpH)2·H2O [M = Fe, Co, Mn, Zn; hedp = C(CH3)(OH)(PO3)2]. Inorg. Chem. 2005, 44, 2761–2765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-C.; Bao, S.-S.; Zheng, L.-M. Ladder-like metal diphosphonates exhibiting field-induced magnetic transitions. Inorg. Chem. Commun. 2007, 10, 1063–1066. [Google Scholar] [CrossRef]
- Zhang, Z.-C.; Gao, S.; Zheng, L.-M. Cobalt diphosphonate with a new double chain structure exhibiting field-induced magnetic transition. Dalton Trans. 2007, 4681–4684. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-H.; Knowles, E.S.; Pajerowski, D.M.; Xia, J.-S.; Yin, L.; Gao, S.; Meisel, M.W.; Zheng, L.-M. Metal Monophosphonates M{(2-C5H4NO)CH2PO3}(H2O)2 (M = Co, Ni, Mn, Cd): Synthesis, Structure, and Magnetism. Inorg. Chem. 2010, 49, 8474–8480. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, M.; Tang, X.; Yuan, R.; Ma, Y. A one-dimensional cobalt phosphonate showing field-induced magnetic transition. Inorg. Chem. Commun. 2018, 89, 60–63. [Google Scholar] [CrossRef]
- Wang, P.; Duan, Y.; Zheng, L. One-dimensional metal phosphonates based on 6-phosphononicotinic acid: A structural and magnetic study. Sci. China Chem. 2010, 53, 2112–2117. [Google Scholar] [CrossRef]
- Cao, D.-K.; Xiao, J.; Li, Y.-Z.; Clemente-Juan, J.M.; Coronado, E.; Zheng, L.-M. Metal Phosphonates Based on {[(Benzimidazol-2-ylmethyl)imino]bis(methylene)}bis(phosphonic Acid): Syntheses, Structures and Magnetic Properties of the Chain Compounds [M{(C7H5N2)CH2N(CH2PO3H)2}](M = Mn, Fe, Co, Cu, Cd). Eur. J. Inorg. Chem. 2006, 2006, 1830–1837. [Google Scholar] [CrossRef]
- Han, G.-F.; Luo, H.-Z.; Ye, Q.; Xiong, R.-G. Two Novel Cobalt(II) Coordination Polymers Based on the Herbicide Glyphosate as a Building Block and their Magnetic Properties. Z. Anorg. Allg. Chem. 2008, 634, 1991–1995. [Google Scholar] [CrossRef]
- Sun, Z.-M.; Prosvirin, A.V.; Zhao, H.-H.; Mao, J.-G.; Dunbar, K.R. New type of single chain magnet based on spin canting in an antiferromagnetically coupled Co(II) chain. J. Appl. Phys. 2005, 97, 10B305. [Google Scholar] [CrossRef]
- Palii, A.V.; Ostrovsky, S.M.; Klokishner, S.I.; Reu, O.S.; Sun, Z.-M.; Prosvirin, A.V.; Zhao, H.-H.; Mao, J.-G.; Dunbar, K.R. Origin of the Single Chain Magnet Behavior of the Co(H2L)(H2O) Compound with a 1D Structure. J. Phys. Chem. A 2006, 110, 14003–14012. [Google Scholar] [CrossRef] [PubMed]
- Caneschi, A.; Gatteschi, D.; Lalioti, N.; Sangregorio, C.; Sessoli, R.; Venturi, G.; Vindigni, A.; Rettori, A.; Pini, M.G.; Novak, M.A. Cobalt(II)-Nitronyl Nitroxide Chains as Molecular Magnetic Nanowires. Angew. Chem. Int. Ed. 2001, 40, 1760–1763. [Google Scholar] [CrossRef]
- Caneschi, A.; Gatteschi, D.; Lalioti, N.; Sessoli, R.; Sorace, L.; Tangoulis, V.; Vindigni, A. Ising-Type Magnetic Anisotropy in a Cobalt(II) Nitronyl Nitroxide Compound: A Key to Understanding the Formation of Molecular Magnetic Nanowires. Chem. Eur. J. 2002, 8, 286–292. [Google Scholar] [CrossRef]
- Clérac, R.; Miyasaka, H.; Yamashita, M.; Coulon, C. Evidence for Single-Chain Magnet Behavior in a MnIII−NiII Chain Designed with High Spin Magnetic Units: A Route to High Temperature Metastable Magnets. J. Am. Chem. Soc. 2002, 124, 12837–12844. [Google Scholar] [CrossRef] [PubMed]
- Lescouëzec, R.; Vaissermann, J.; Ruiz-Pérez, C.; Lloret, F.; Carrasco, R.; Julve, M.; Verdaguer, M.; Dromzee, Y.; Gatteschi, D.; Wernsdorfer, W. Cyanide-Bridged Iron(III)–Cobalt(II) Double Zigzag Ferromagnetic Chains: Two New Molecular Magnetic Nanowires. Angew. Chem. 2003, 115, 1521–1524. [Google Scholar] [CrossRef]
- Miyasaka, H.; Clérac, R.; Mizushima, K.; Sugiura, K.; Yamashita, M.; Wernsdorfer, W.; Coulon, C. [3Mn2(saltmen)2Ni(pao)2(L)2](A)2 with L = Pyridine, 4-Picoline, 4-tert-Butylpyridine, N-Methylimidazole and A = ClO4−, BF4−, PF6−, ReO4−: A Family of Single-Chain Magnets. Inorg. Chem. 2003, 42, 8203–8213. [Google Scholar] [CrossRef] [PubMed]
- Coulon, C.; Clérac, R.; Lecren, L.; Wernsdorfer, W.; Miyasaka, H. Glauber dynamics in a single-chain magnet: From theory to real systems. Phys. Rev. B 2004, 69, 132408. [Google Scholar] [CrossRef]
- Gatteschi, D.; Sessoli, R. Quantum Tunneling of Magnetization and Related Phenomena in Molecular Materials. Angew. Chem. Int. Ed. 2003, 42, 268–297. [Google Scholar] [CrossRef] [PubMed]
- Gatteschi, D.; Sessoli, R.; Villain, J. Mesoscopic Physics and Nanotechnology. In Molecular Nanomagnets; Oxford University Press: Oxford, UK, 2006; ISBN 978-0-19-856753-0. [Google Scholar]
- Sheldrick, G.M. Twinabs; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; Giacovazzo, C.; Mallamo, M.; Mazzone, A.; Polidori, G.; Spagna, R. SIR2011: A new package for crystal structure determination and refinement. J. Appl. Crystallogr. 2012, 45, 357–361. [Google Scholar] [CrossRef]
- Václav, P.; Michal, D.; Lukáš, P. Crystallographic Computing System JANA2006: General features. zkri 2014, 229, 345. [Google Scholar] [CrossRef]
- Shannon, R.D.; Prewitt, C.T. Effective ionic radii in oxides and fluorides. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1969, 25, 925–946. [Google Scholar] [CrossRef] [Green Version]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Spackman, M.A.; Mitchell, A.S. Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Crystallogr. Sect. B 2004, 60, 627–668. [Google Scholar] [CrossRef] [PubMed]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Bloyet, C.; Rueff, J.-M.; Caignaert, V.; Lohier, J.-F.; Cardin, J.; Jaffrès, P.-A.; Raveau, B. Fluorenyl Zinc Phosphonate Zn(H2O)PO3–C13H9·H2O: Hybrid Columnar Structure with Strong C–H···π Interactions. Z. Anorg. Allg. Chem. 2017, 643, 250–255. [Google Scholar] [CrossRef]
- Demadis, K.D.; Panera, A.; Anagnostou, Z.; Varouhas, D.; Kirillov, A.M.; Císařová, I. Disruption of “Coordination Polymer” Architecture in Cu2+ Bis-Phosphonates and Carboxyphosphonates by Use of 2,2′-Bipyridine as Auxiliary Ligand: Structural Variability and Topological Analysis. Cryst. Growth Des. 2013, 13, 4480–4489. [Google Scholar] [CrossRef]
- Bloyet, C.; Rueff, J.-M.; Cardin, J.; Caignaert, V.; Doualan, J.-L.; Lohier, J.-F.; Jaffrès, P.-A.; Raveau, B. Excimer and Red Luminescence Due to Aggregation-Induced Emission in Naphthalene Based Zinc Phosphonate. Eur. J. Inorg. Chem. 2018, 3095–3103. [Google Scholar] [CrossRef]
- Carlin, R.L. Magnetochemistry; Springer: Berlin/Heidelberg, Germany, 1986; ISBN 978-3-642-70735-3. [Google Scholar]
- Goodenough, J.B. An interpretation of the magnetic properties of the perovskite-type mixed crystals La1−xSrxCoO3−λ. J. Phys. Chem. Solids 1958, 6, 287–297. [Google Scholar] [CrossRef]
- Kanamori, J. Superexchange interaction and symmetry properties of electron orbitals. J. Phys. Chem. Solids 1959, 10, 87–98. [Google Scholar] [CrossRef]
- Oka, Y.; Inoue, K.; Kumagai, H.; Kurmoo, M. Long-Range Magnetic Ordering at 5.5 K for Cobalt(II)–Hydroxide Diamond Chains Isolated by 17 Å with α-Phenylcinnamate. Inorg. Chem. 2013, 52, 2142–2149. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Turner, M.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer; University of Western Australia: Crawley, WA, Australia, 2012. [Google Scholar]








| Formula | Co(H2O)2PO3C–C12H9·H2O |
|---|---|
| FW | 357.20 |
| Space group | P21/c |
| a (Å) | 15.216(3) |
| b (Å) | 4.827(8) |
| c (Å) | 19.590(3) |
| α, β, γ (°) | 90, 107.160(4), 90 |
| Z | 4 |
| V (Å3) | 1375.0(4) |
| dcalc (g/cm3) | 1.716 |
| µ (mm−1) | 1.389 |
| Radiation source λ (Å) | Mo Kα 0.71069 |
| Pattern range 2Ѳ (°) | 2.8–66.94 |
| No. of reflexions | 21,476 |
| No. of soft constraints | 0 |
| Weighted R factor | 0.0877 |
| R[F2 > 2σ(F2)] | 0.0462 |
| Rint (internal R-value) | 0.0371 |
| S (Goodness of the fit) | 1.720 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bloyet, C.; Rueff, J.-M.; Perez, O.; Pautrat, A.; Caignaert, V.; Raveau, B.; Rogez, G.; Jaffrès, P.-A. One-Dimensional Fluorene-Based Co(II) Phosphonate Co(H2O)2PO3C–C12H9·H2O: Structure and Magnetism. Inorganics 2018, 6, 93. https://doi.org/10.3390/inorganics6030093
Bloyet C, Rueff J-M, Perez O, Pautrat A, Caignaert V, Raveau B, Rogez G, Jaffrès P-A. One-Dimensional Fluorene-Based Co(II) Phosphonate Co(H2O)2PO3C–C12H9·H2O: Structure and Magnetism. Inorganics. 2018; 6(3):93. https://doi.org/10.3390/inorganics6030093
Chicago/Turabian StyleBloyet, Clarisse, Jean-Michel Rueff, Olivier Perez, Alain Pautrat, Vincent Caignaert, Bernard Raveau, Guillaume Rogez, and Paul-Alain Jaffrès. 2018. "One-Dimensional Fluorene-Based Co(II) Phosphonate Co(H2O)2PO3C–C12H9·H2O: Structure and Magnetism" Inorganics 6, no. 3: 93. https://doi.org/10.3390/inorganics6030093
APA StyleBloyet, C., Rueff, J. -M., Perez, O., Pautrat, A., Caignaert, V., Raveau, B., Rogez, G., & Jaffrès, P. -A. (2018). One-Dimensional Fluorene-Based Co(II) Phosphonate Co(H2O)2PO3C–C12H9·H2O: Structure and Magnetism. Inorganics, 6(3), 93. https://doi.org/10.3390/inorganics6030093