1. Introduction
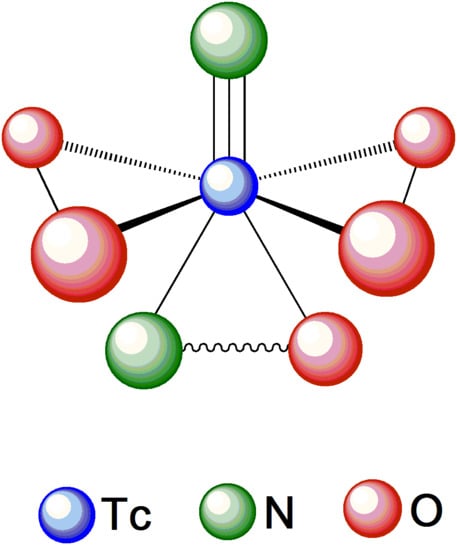
The anionic technetium complex [
99gTc][Tc(N)(O
2)
2Cl]
−, reported by Baldas and coworkers a few decades ago [
1], was the first example of a transition compound containing a metal–nitrido multiple bond (M≡N) tethered to a peroxo (O
22−) group. In this remarkable compound, the oxidation state of the technetium atom is +7 and the resulting coordination arrangement manifests high solubility and stability in aqueous solution. Previous studies have reported only a few reactions involving the [
99gTc][Tc(N)(O
2)
2] fragment. In these reactions, a bidentate ligand L (L = 2,2′-bipyridine, 1,10-phenantroline, oxalic acid) coordinated the metal atom without disrupting the [
99gTc][Tc(N)(O
2)
2] metallic moiety to yield monosubstituted [
99gTc][Tc(N)(O
2)
2(L)] or oxalate-bridged, dinuclear [
99gTc][{Tc(N)(O
2)
2}
2(L’)]
2− (L’ = dianionic oxalate) complexes [
2].
Despite its interesting properties, the chemistry of the Tc(VII) nitrido-peroxo functional group still remains poorly investigated. Several potential advantages could be envisaged by exploiting the distinctive chemical features of the [99gTc][Tc(N)(O2)2] fragment, particularly in an aqueous solution. As mentioned above, the oxidation state of technetium in [99gTc][Tc(N)(O2)2] is +7, thus identical to that assigned to the same metal in the tetraoxo anion [99gTc][TcO4]−, which is the most stable species of technetium in water. Therefore, it is expected that the [99gTc][Tc(N)(O2)2] moiety should be strongly resistant towards oxidation reactions of the metal center in water and, possibly, only affected by reduction processes that are less common in an aqueous medium. This redox inertness, comparable to that of the tetraoxo anion [99gTc][TcO4]−, might confer a higher stability towards hydrolysis in water to the resulting nitrido-peroxo complexes. However, whereas substitution reactions onto [99gTc][TcO4]− are quite arduous under mild conditions, the [99gTc][Tc(N)(O2)2] moiety apparently displays a higher reactivity. This observation may point towards the use of the [99gTc][Tc(N)(O2)2] group as a convenient synthon for the preparation of technetium complexes in full aqueous conditions.
Besides the obvious interest in further expanding the knowledge of technetium chemistry [
3,
4,
5,
6,
7,
8,
9,
10], there exists a practical interest in exploring the properties of the Tc(VII) nitrido-peroxo core that is related to the use of technetium complexes as diagnostic imaging agents for single photon emission tomography (SPECT) [
11,
12,
13,
14]. A crucial requirement for the preparation of an injectable diagnostic radiopharmaceutical radiolabeled with the γ-emitting radioisotope Tc-99m is that the reaction should be carried out under strictly controlled physiological conditions. As previously noted, the observed high water solubility and redox inertness of the [
99gTc][Tc(N)(O
2)
2] synthon suggests that it could be conveniently utilized for the development of novel classes of Tc-99m radiopharmaceuticals.
The present study was carried out in two separate steps. Initially, the reactivity of the Tc(VII) nitrido-peroxo core towards ligand substitution was further evaluated by conducting simple exchange reactions onto the [99gTc][Tc(N)(O2)2] group using a number of natural amino acids. Then, a procedure for obtaining the functional moiety [99mTc][Tc(N)(O2)2] radiolabeled with the nuclear isomer Tc-99m, at the nanomolar concentration scale typically employed in radiopharmaceutical preparations, was investigated. As a preliminary application of this novel chemistry to the labeling of biologically relevant molecules, the radiolabeling of the antibody trastuzumab was accomplished by the simple mixing of the [99mTc][Tc(N)(O2)2] precursor with the protein.
2. Results
Addition of the amino acids proline (HPro), glycine (HGly) and alanine (HAla) to an acetonitrile solution of [
99gTc][Tc(N)(O
2)
2X]
− (X = Cl, Br), in the presence aqueous boric acid, [B(OH)
3], yielded the new complexes of the general formula [
99mTc][Tc(N)(O
2)
2(L)]
− (where L indicates the deprotonated carboxylate form of the corresponding amino acid) in low yield. The resulting bright-orange compounds were recovered from the reaction solution as salts of the bulky cation [As(C
6H
5)
4]
+ by slow evaporation under an argon stream. When [B(OH)
3] was not added to the mixture, no reaction was observed, a result which suggests that, presumably, the role of this reagent in the process was as halogen (Cl
−, Br
−) acceptor from the precursor complex. A higher yield was achieved following another synthetic route originally proposed by Baldas and coworkers [
2]. This procedure first required the in situ generation of nitrido pertechnic acid, [
99mTc][Tc(N)(OH)
3]
n, by aqueous hydrolysis of the nitrido complex [
99gTc][Tc(N)Cl
5]
− [
15,
16]. The subsequent addition of H
2O
2 led to the formation of an hypothetical nitrido-peroxo technetic acid, which was further reacted with excess of the appropriate amino acid to yield the final substituted complexes [
99mTc][Tc(N)(O
2)
2(L)]
− (L = deprotonated Pro, Gly and Ala).
Figure 1 illustrates a schematic drawing of these reactions.
The chemical composition of the resulting complexes was inferred from data of elemental analyses and mass spectra. Proton magnetic resonance (PMR) spectra of the complexes are more complex than those of the free amino acids and a detailed assignment of all protons in these spectra was not carried out in the present study. However, detection of proton signals provided strong evidence of the presence of the coordinated amino acid. The spectra of the complexes containing the simplest amino acids, glycine and alanine, were easier to interpret. In free glycine, the two protons attached to the α-carbon were equivalent and the PMR spectrum showed a sharp singlet. Upon binding the Tc≡N group, these two protons lost their equivalence, presumably because of the two possible, syn and anti, orientations these protons can achieve with respect to the Tc≡N multiple bond. As a result, the PMR spectrum transformed in a broader multiplet, probably generated by the combination of two doublets, each corresponding to a single proton. Conversely, the PMR of the alanine derivative approximately retained the overall structure of the free ligand because of the presence of an asymmetrical α-carbon bound to a single hydrogen and one methyl group. As a result, the spectrum was roughly composed of a doublet and of a pseudo-quartet assigned to the methyl group and the hydrogen atom bound to the α-carbon, respectively. It should be noted that other simple amino acids were tested for their reactivity towards the [99gTc][Tc(N)(O2)2] moiety and, invariably, the formation of various chemical species was observed. However, despite many efforts, it was not possible to isolate these products in a sufficiently purified form to obtain a satisfactory chemical characterization.
Mass spectroscopy was particularly informative. The electrospray ionization mass spectra (ESI-MS) collected in both positive- and negative-ion modes for all complexes exhibited the same general pattern (
Supplementary Materials). In positive-ion mode, the signals corresponding to the complexes were not detected, but only those associated with the protonated free amino acids, the [
99gTc][Tc(N)(O
2)
2] fragment and the counter cation [As(C
6H
5)
4]
+, were observed. Conversely, negative-ion mode ESI-MS showed the main complex ions and the deprotonated free amino acids. These results provide strong evidence supporting the proposed chemical formulation for these complexes.
Infrared spectra (FT-IR) of the complexes clearly showed the diagnostic strong band, in the interval 1050–1060 cm−1, associated with the ν(Tc≡N) stretching vibration.
It was more challenging to replicate this chemistry under the experimental conditions usually employed for the preparation of Tc-99m radiopharmaceuticals, where the concentration of the radionuclide ranges between 10
−9 to 10
−12 mol dm
−3 (no-carrier-added level = n.c.a., also called “tracer level”). The selected approach was grounded in the results obtained at the macroscopic level. The formation of the
99mTc nitrido-peroxo fragment, [
99mTc][Tc(N)(O
2)
2], was attempted, starting from a pre-formed
99mTc nitrido intermediate, which was further reacted with H
2O
2 in low concentrations. Baldas and coworkers reported a procedure for preparing the halogeno-nitrido complex [
99mTc][Tc(N)Cl
4]
− at n.c.a. level through the reaction of sodium azide (NaN
3) with [
99mTc][TcO
4]
− in the presence of the corresponding halogenidric acid (HCl) [
17]. Subsequently, it was found that this procedure was rather cumbersome and difficult to apply to the preparation of Tc-99m nitrido compounds at tracer level as it required an evaporation step. To ensure the formation of the Tc≡N multiple bond, a more efficient approach was proposed based on the reaction of [
99mTc][TcO
4]
− with
N-methyl-
S-methyl-dithiocarbazate [DTC = H
3C−NH−NH−C(=S)SCH
3], promoted by the addition of an appropriate reducing agent (tertiary phosphines or SnCl
2). This reaction led to a mixture of different species that were demonstrated to invariably incorporate the Tc≡N triple bond [
18,
19]. In this study, therefore, it was very convenient to utilize these species as pre-formed nitrido intermediates for the production, at tracer level, of the supposed [
99mTc][Tc(N)(O
2)
2] moiety by simple addition of a source of hydrogen peroxide to the mixture. In fact, the addition of a few microliters of H
2O
2 had a dramatic impact on the profile of the high-performance liquid chromatography (HPLC) chromatogram of the starting mixture, thus indicating that some reaction had occurred. However, no single product was identified by HPLC and only a complicated overlap of different peaks was observed. In an attempt to further characterize this mixture of chemical species, Ultra HPLC (UPLC) was utilized, following the same methods described previously for the analysis of
99mTc radiopharmaceuticals [
20]. This more powerful chromatographic technique allowed us to achieve a sharper separation of the various products (
Supplementary Materials), but without providing additional hints on their chemical nature. However, convincing evidence that all species still included a Tc≡N multiple bond came from the results of the reaction of the mixture with excess of the sodium salt of the ligand diethyldithiocarbamate {DEDC = [(CH
3CH
2)
2NC(=S)S]
−}, which is a common reagent avidly binding the Tc≡N group. When this ligand was added to the freshly prepared mixture, in combination with some amount of SnCl
2, the well-characterized bis-substituted, nitrido complex [
99mTc][Tc(N)(DEDC)
2] [
21] was isolated in high yield (77% calculated on the initial
99mTc activity) and characterized by UPLC (
Supplementary Materials).
An unexpected practical application of this chemistry spawned from the observation that the mixture of Tc-99m species, generated by the addition of peroxides, showed a high reactivity towards proteins. More precisely, it was found that a simple mixing of the solution containing the hypothetical mixture of 99mTc-nitrido-peroxo species with a lyophilized formulation of the antibody trastuzumab allowed for the obtaining of high radiolabeling yields (>90%) of this protein. After flushing the radioactive solution with an argon stream, the procedure was extremely easy to apply, as it did not require any preliminary chemical treatment of the antibody for appending to its structure a chelating group for the radiometal.
3. Discussion
This work was aimed at rediscovering a class of technetium complexes, first described decades ago by Baldas and coworkers, that was subsequently almost completely abandoned. A characteristic feature of these complexes is that they contain a technetium atom in some of its highest oxidation states (+6 and +7) and coordinated to a nitrido nitrogen [N3−] group, thus forming a remarkably stable terminal Tc≡N multiple bond. A relevant example of this type of compounds was provided by complexes containing the nitrido-peroxo moiety, [99gTc(N)(O2)2], a metallic fragment that can be prepared by the reaction of nitrido technetic(VI) acid, [99gTc][Tc(N)(OH)3]n, with hydrogen peroxide. This moiety exhibits high inertness towards hydrolysis in water and, therefore, could be of interest for developing a new category of 99mTc radiopharmaceuticals. However, despite the ability of the nitrido substituent to stabilize the highest oxidation states of technetium, this chemistry was not further explored and only a few complexes comprising the [99gTc(N)(O2)2] fragment have been reported. In particular, substituted complexes of the type [99gTc(N)(O2)2(L)] where L represents the neutral bidentate ligands 2,2′-bipyridyl (Bipy) and 1,10-phenantroline (Phen), were synthesized and characterized.
Inspired by these results, the present work was undertaken to investigate the reactivity of the [
99gTc(N)(O
2)
2] synthon towards simple amino acids. The starting precursors [
99gTc(N)(O
2)
2X]
− (X = Cl, Br) was reacted with the amino acids alanine (HAla), glycine (HGly) and proline (HPro) to yield the corresponding monoanionic complexes [
99gTc(N)(O
2)
2(L)]
−, where L stands for the deprotonated form of these three amino acids. A higher yield was obtained by using nitrido pertechnic acid, [
99gTc][Tc(N)(O
2)(OH)
2], as the starting material. The structure of the resulting complexes was inferred from analytical data (
Supplementary Materials), which supported the hypothesis that, in these complexes, the amino acid acts as a bidentate ligand binding the metal through the deprotonated carboxylic oxygen atom and the neutral amino nitrogen atom. Tentatively, a final pentagonal-bipyramidal geometry could be assigned to these products comprising an apical Tc≡N group and two equatorial dioxo ligands as observed in the oxalate-bridged Tc(VII) nitrido-peroxo dimer [
99gTc][{Tc(N)(O
2)
2}
2(Ox)]
2− (Ox = [C
2O
4]
4−) [
22].
The data collected at the macroscopic level with the long-lived isotope
99gTc led us to speculate that the observed reactivity of the [
99gTc][Tc(N)(O
2)
2] synthon towards simple amino acids could be exploited for radiolabeling amino acid-rich biomolecules, such as proteins and antibodies, with the diagnostic γ-emitting radioisotope
99mTc. To test this hypothesis, a procedure for producing the Tc(VII) nitrido-peroxo moiety at tracer level (concentration of
99mTc below 10
−9 mol dm
−3) using the γ-emitting radionuclide
99mTc was attempted with partially satisfactory results. This procedure was based on a well-established method for producing the Tc≡N bond at tracer level [
18,
19], which was complemented by the addition of hydrogen peroxide to the solution containing the intermediate
99mTc nitrido precursor. Evidence that the [
99gTc][Tc(N)(O
2)
2] moiety formed at this very low concentration scale was obtained only indirectly, as chromatographic analysis revealed the production of a complicate mixture of compounds (
Supplementary Materials). No attempts have been pursued to further characterize the chemical nature of the components of this mixture that, apparently, are involved in complex equilibria in solution. However, the addition to this mixture of the ligand diethyl-dithiocarbamate (DEDC = [(CH
3CH
2)
2NCS
2]
−), in combination with the reducing agent (SnCl
2), led to the formation of the well-known Tc(V) nitrido complex [
99mTc][Tc(N)(DEDC)
2), thus suggesting that the Tc≡N bond was preserved in these reactions.
A preliminary labeling study was carried out using the antibody trastuzumab, which was simply mixed with an aliquot of the solution containing the mixture of the products supposedly containing the [
99mTc(N)(O
2)
2] moiety. The results were particularly promising, as the reaction occurred at room temperature within a few minutes, and the measured labeling yield was almost quantitative. This application is noteworthy because it could potentially allow for the labeling of proteins without the need to append any ancillary chelating group to the antibody, as is usually necessary for tethering the radiometal to a biomolecule [
13,
14]. However, it should be emphasized that this observation has to be confirmed by further, larger studies involving different types of antibodies to evaluate their stability and retention of immunoreactivity after reaction with the radiometal fragment. In fact, it could be speculated that the observed high labeling yield originated from variations in the chemical composition of the various
99mTc species present in the reaction mixture and that these modifications might allow them to attach to the protein at different binding sites. Although this behavior could be beneficial for increasing the labeling yield, it may cast some concern about the preservation of the antibody’s native biological properties.
4. Materials and Methods
Caution: Technetium-99g is a weak β−-emitter (Eβ = 0.292 MeV, t1/2 = 2.12 × 105 years). All manipulations were carried out in a radiochemistry laboratory approved for low-level radioactivity using HEPA-filtered monitored fume hoods and gloveboxes. When handled in milligram amounts, 99gTc does not present a serious health hazard since common laboratory glassware provides adequate shielding. Bremsstrahlung is not a significant problem due to the low energy of the β-particles. However, normal radiation safety procedures must be used at all times, especially with solid samples, to prevent contamination and inhalation.
The common laboratory solvents were reagent grade and used as purchased. Hydrogen peroxide (H
2O
2, 30%
w/
w in H
2O), boric acid (H
3BO
3, ACS reagent), tetraphenylarsonium chloride hydrate ([As(C
6H
5)
4]Cl, 97%) and the sodium salt of dietyldithiocarbamate were purchased from Merck, Darmstadt, Germany. Technetium-99g as [
99gTc]TcO
4][NH
4] was obtained from Oak Ridge National Laboratory (Oak Ridge, TN, USA). The samples were dissolved in water and treated with excess aqueous ammonia and H
2O
2 (30%
w/
w) at 80 °C prior to use to eliminate residual [
99gTc]TcO
2. Solid [
99gTc][TcO
4][NH
4] was precipitated by slow evaporation under heating at 40 °C and then re-dissolved in water and treated with aqueous KOH to yield crystals of purified [
99gTc][TcO
4]K by controlled evaporation under a nitrogen stream. The complexes [
99gTc][Tc(N)X
4][As(C
6H
5)
4] (X = Cl, Br) [
23,
24,
25,
26] and the precursor complex [
99gTc][Tc(N)Cl
5]Cs
2 [
27] were prepared by literature methods. Technetium-99m as [
99mTc][TcO
4]Na was withdrawn from a Drytec™
99Mo/
99mTc generator (GE Healthcare) by elution with a physiological solution.
The antibody trastuzumab (Herceptin
®) [
28,
29] was received from Hoffmann-La Roche (Basel, Switzerland). Before usage, the samples were dissolved in water and purified by passing through an Ultra-4, 30-kDa column (Amicon
®, Sigma Aldrich, St Louis, MO, USA) in six cycles and then isolated using a centrifuge (HuMax 4k, Human, Wiesbaden, Germany). The concentration of trastuzumab was adjusted to 1.0 mg/mL using a spectrophotometer (6715 nm UV/Vis, Jenway
®, Staffordshire, UK). A modified two-day protocol [
30] was applied for the freeze-drying of the antibody, using a Free Zone Stoppering Tray Dryer (Labconco, Kansas City, MO, USA).
Elemental analyses (C, H, N, S) were performed on a Carlo Erba 1106 elemental analyzer. The analysis of the radioactive technetium-99g was performed as previously described by dissolving weighed samples in HNO3/H2O2 mixtures and counting the resulting radioactivity in a Packard TRICARB 3000 scintillation counter. The infrared spectra were recorded on an FT-IR-6000 Spectrometer (Jasko, Easton, MD, USA). The proton NMR spectra were collected on a Bruker Advance 500 (Bruker BioSpin GmbH, Germany). The electrospray ionization mass spectra (ESI-MS) of technetium compounds, in the positive- and negative-ion mode (ca. 10−6 mol dm−3 in methanol solutions), were recorded on a Bruker Esquire HCT (Bruker Daltonics, Billerica, MA, USA). Instant thin-layer chromatography (ITLC) was performed on silica-gel (SG) strips, which were subsequently analyzed with a C431200 Cyclone Plus Storage Phosphor Imager equipped with OptiQuant software (Perkin-Elmer, Shelton CT, Beaconsfield, England) to locate radioactive spots on the surface. High-performance liquid chromatography (HPLC) was performed on a Beckman System Gold instrument equipped with a programmable solvent model 126, a sample injection valve 210A, a scanning detector Module 166, and a radioisotope detector model 170. The HPLC analyses were carried out using a reversed-phase Agilent precolumn Zorbax 300SB-C18 (4.6 × 12.5 mm) and a reversed-phase Agilent column Zorbax 300SB- C18 (4.6 × 250 mm) at a flow rate of 1.0 mL/min. Ultra HPLC (UPLC) was performed with a ACQUITY UPLC system (Waters, Milford, MA, USA), equipped with an autoinjector, UV detector, and gamma flow count detector (Bioscan, Washington, DC, USA) using a Waters ACQUITY UPLC® BEH column (1.7 mm × 2.1 0 mm). The data collection was accomplished using the Chromeleon chromatography software package.
4.1. Synthesis of the Complexes
The complexes [99gTc][Tc(N)(O2)2(L)] [As(C6H5)4] (L = Pro, Gly, Ala) were prepared following two different synthetic routes.
4.1.1. Method (a)
The complexes [
99gTc][Tc(N)(O
2)
2X][As(C
6H
5)
4] (X = Cl, Br) were prepared as reported previously [
2]. These complexes (0.300 mmol) were dissolved in the minimum volume of CH
3CN. To the resulting solution, 0.30 mL of an aqueous solution of boric acid (0.1 M) were added and the color turned to pale brown. Then, the ligand L (0.600 mmol) was added to the mixture. Upon standing, the color of the solution darkened slowly. A bright orange precipitate was collected by the slow evaporation of the solvents under an argon stream. The solid was isolated by filtration and washed with water and diethyl ether and dried under vacuum. Yield, 23% (X = Cl), 18% (X = Br).
4.1.2. Method (b)
Polymeric nitridotechnetic(VI) acid, [
99gTc][Tc(N)(OH)
3]
n, was prepared as described previously by the hydrolysis of Cs
2[
99gTc][Tc(N)Cl
5] in water and isolated as a brown precipitate [
1,
15,
16,
27]. The brown solid (84 mg, 0.15 mmol) was dissolved in 0.5 mL of 10%
w/
w H
2O
2, followed by the addition of an excess of the ligand L (0.80 mmol) to yield a pale-yellow solution, which was slightly heated at 30 °C for 15 min. Addition of [As(C
6H
5)
4]Cl (167.52 mg, 0.40 mmol) caused the precipitation of an orange solid, which was collected by filtration, washed with water and diethyl ether, and then dried under vacuum. Yield, 57%.
4.2. Analytical Characterization
This paragraph reports the most relevant properties of the complexes described in this work. A more detailed description of the synthetic and analytical methods, the antibody’s labeling procedures and the determination of the radiochemical purity of the resulting radiolabeled conjugate is reported in the
Supplementary Materials.
[As(C6H5)4]{[99gTc]Tc(N)(O2)2(Pro)]}. Empirical formula: [C29H28N2O6AsTc]. Elemental analysis [%found (%calculated)]: C, 51.93 (51.64); H, 4.66 (4.18); N, 4.89 (4.15); Tc, 13.95 (14.68). ESI-MS (m/z, negative): [M]− calculated for [C5H8N2O6Tc] 291.13, found, 291.05. RP-UPLC: retention time (tR) = 1.66 min. IR (cm−1, KBr): 1050, ν(Tc≡N).
[As(C6H5)4]{[99gTc]Tc(N)(O2)2(Gly)]}. Empirical formula: [C26H24N2O6AsTc]. Elemental analysis [%found (%calculated)]: C, 50.16 (49.22); H, 4.01 (3.81); N, 4.77 (4.42); Tc, 14.95 (15.60). ESI-MS (m/z, negative): [M]− calculated for [C2H4N2O6Tc] 251.06, found 251.00. RP-UPLC: retention time (tR) = 1.43 min. IR (cm−1, KBr): 1055, ν(Tc≡N).
[As(C6H5)4]{[99gTc]Tc(N)(O2)2(Ala)]}. Empirical formula: [C27H26N2O6AsTc]. Elemental analysis [%found (%calculated)]: C, 50.81 (50.01); H, 4.35 (4.04); N, 4.53 (4.32); Tc, 14.88 (15.27). ESI-MS (m/z, negative): [M]− calculated for [C3H6N2O6Tc] 265.09, found 265.02. RP-UPLC: retention time (tR) = 1.52 min. IR (cm−1, KBr): 1053, ν(Tc≡N).
As a representative example, the ESI-MS and UPLC chromatogram of the complex [As(C
6H
5)
4]{[
99gTc]Tc(N)(O
2)
2(Gly)]} are reported in
Figure 2 below. The complexes are soluble in CH
3CN, CHCl
3 and CH
2Cl
2. When left to stand in non-aqueous solvents and under an argon atmosphere, their chromatographic profiles did not change significantly over time. However, when non-anhydrous conditions were used or a few microliters of H
2O were added to the solutions, some decomposition was observed (approximately, 10% after 5 h by HPLC analysis) with the formation of different species. The nature of these mixtures was not further investigated.
5. Conclusions
A decade-old technetium chemistry, which was not fully explored in the past, has been rediscovered in the present study and first evaluated as an efficient tool for producing new classes of complexes. The characteristic motif permeating the molecular structure of these complexes is formed by the nitrido-peroxo moiety [99gTc][Tc(N)(O2)2], where a terminal Tc(VII) nitrido core is bound to two peroxo groups. The resulting metallic fragment exhibits an unexpectedly high stability in water, thus indicating that the nitrido nitrogen atom is particularly effective in stabilizing the metal in its highest oxidation states, presumably because of its remarkable π-donor character. As a first attempt to evaluate the reactivity of the [99gTc][Tc(N)(O2)2] metallic synthon towards simple molecules, the reaction of some nitrido-peroxo precursors with natural amino acids was investigated. It was observed that the simple addition of the selected amino acid to a solution of the [99gTc][Tc(N)(O2)2] fragment elicited a reaction, although it was difficult to isolate pure products from the reaction mixture. Only with the amino acids alanine, glycine and proline was it possible to recover products sufficiently pure to allow for their chemical characterization. Yet, these results, combined with the high stability in aqueous solution of the [99gTc][Tc(N)(O2)2] group, motivated the extension of this study to investigate whether it was possible to prepare this class of complexes at the tracer level with the metastable γ-emitting radioisotope 99mTc. This application would be remarkable because of the important role played by 99mTc radiopharmaceuticals in diagnostic imaging. Albeit these preliminary results should be confirmed by further studies, experimental evidence suggests that the [99mTc][Tc(N)(O2)2] moiety can be generated at the low concentration scale typical of 99mTc preparations. Most interestingly, this precursor, when mixed with the antibody trastuzumab, reacted quickly and in high yield, thus unearthing an unexpected route for the efficient labeling of large proteins with 99mTc.
Despite the chemistry of Tc nitrido-peroxo complexes being discovered many years ago, the interest in this uncommon category of compounds faded away rapidly and their synthesis was not actively pursued. The present work might contribute to stimulating further investigations in this field and, possibly, opening new avenues in technetium chemistry.