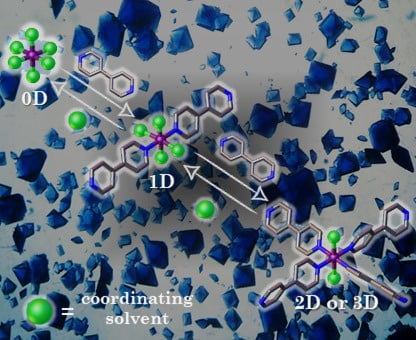
Effect of Coordinating Solvents on the Structure of Cu(II)-4,4′-bipyridine Coordination Polymers
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Synthesis
4.1.1. Synthesis of {[Cu(bpy)2(H2O)2](ClO4)2·2DMA}n (3)
4.1.2. Synthesis of {[Cu(bpy)2(DMF)2](ClO4)2·2(bpy)}n (4) and {[Cu(bpy)2(H2O)2](ClO4)2·2(bpy)·2(H2O)2}n (5)
4.1.3. Synthesis of {[Cu(bpy)2(DMF)(DMSO)](ClO4)2}n (6)
4.2. Crystal Structure Determination
4.3. Powder X-ray Diffraction (PXRD)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional Porous Coordination Polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal–Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.; Schön, J.C. “Design” in Chemical Synthesis—An Illusion? Angew. Chem. Int. Ed. 2006, 45, 3406–3412. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, M. Design of MOFs and intellectual content in reticular chemistry: A personal view. Chem. Soc. Rev. 2009, 38, 1215–1217. [Google Scholar] [CrossRef]
- Goesten, M.G.; Kapteijn, F.; Gascon, J. Fascinating chemistry or frustrating unpredictability: Observations in crystal engineering of metal–organic frameworks. CrystEngComm 2013, 15, 9249–9257. [Google Scholar] [CrossRef]
- Yaghi, O.M. Reticular Chemistry—Construction, Properties, and Precision Reactions of Frameworks. J. Am. Chem. Soc. 2016, 138, 15507–15509. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef]
- Deng, H.; Grunder, S.; Cordova, K.E.; Valente, C.; Furukawa, H.; Hmadeh, M.; Gándara, F.; Whalley, A.C.; Liu, Z.; Asahina, S.; et al. Large-pore apertures in a series of metal–organic frameworks. Science 2012, 336, 1018–1023. [Google Scholar] [CrossRef]
- Noro, S.; Kitaura, R.; Kondo, M.; Kitagawa, S.; Ishii, T.; Matsuzaka, H.; Yamashita, M. Framework Engineering by Anions and Porous Functionalities of Cu(II)/4,4′-bpy Coordination Polymers. J. Am. Chem. Soc. 2002, 124, 2568–2583. [Google Scholar] [CrossRef]
- Carlucci, L.; Cozzi, N.; Ciani, G.; Moret, M.; Proserpio, D.M.; Rizzato, S. A three-dimensional nanoporous flexible network of ‘square-planar’ copper(II) centres with an unusual topology. Chem. Commun. 2002, 1354–1355. [Google Scholar] [CrossRef]
- Blake, A.J.; Hill, S.J.; Hubberstey, P.; Li, W.-S. Rectangular grid two-dimensional sheets of copper(II) bridged by both co-ordinated and hydrogen bonded 4,4′-bipyridine (4,4′-bipy) in [Cu(μ-4,4′-bipy)(H2O)2(FBF3)2]·4,4′-bipy. J. Chem. Soc. Dalt. Trans. 1997, 913–914. [Google Scholar] [CrossRef]
- Masciocchi, N.; Cairati, P.; Carlucci, L.; Mezza, G.; Ciani, G.; Sironi, A. Ab-initio X-ray powder diffraction structural characterization of co-ordination compounds: Polymeric [{MX2(bipy)}n] complexes (M = Ni or Cu; X = Cl or Br; bipy = 4,4′-bipyridyl). J. Chem. Soc. Dalt. Trans. 1996, 2739–2746. [Google Scholar] [CrossRef]
- Rizzato, S.; Moret, M.; Beghi, F.; Lo Presti, L. Crystallization and structural properties of a family of isotopological 3D-networks: The case of a 4,4′-bipy ligand–M2+ triflate system. CrystEngComm 2018, 20, 3784–3795. [Google Scholar] [CrossRef]
- Komori-Orisaku, K.; Hoshino, K.; Yamashita, S.; Koide, Y. Water Molecules as Binders in Transformation of 2D Coordination Polymer [Cu(4,4′-bpy)2(OTf)2]n into Parallel Aligned 3D Architectures. Bull. Chem. Soc. Jpn. 2010, 83, 276–278. [Google Scholar] [CrossRef]
- Kondo, A.; Kajiro, H.; Noguchi, H.; Carlucci, L.; Proserpio, D.M.; Ciani, G.; Kato, K.; Takata, M.; Seki, H.; Sakamoto, M.; et al. Super Flexibility of a 2D Cu-Based Porous Coordination Framework on Gas Adsorption in Comparison with a 3D Framework of Identical Composition: Framework Dimensionality-Dependent Gas Adsorptivities. J. Am. Chem. Soc. 2011, 133, 10512–10522. [Google Scholar] [CrossRef] [PubMed]
- Rancan, M.; Armelao, L. Exploiting dimensional variability in coordination polymers: Solvent promotes reversible conversion between 3D and chiral 1D architectures. Chem. Commun. 2015, 51, 12947–12949. [Google Scholar] [CrossRef]
- Truccolo, G.; Tessari, Z.; Tessarolo, J.; Quici, S.; Armelao, L.; Rancan, M. A Cu(II) metallocycle for the reversible self-assembly of coordination-driven polyrotaxane-like architectures. Dalt. Trans. 2018, 47, 12079–12084. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. IUCr SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]





| Compound | 3 | 4 | 5 | 6 |
|---|---|---|---|---|
| Formula | C28H38Cl2CuN6O12 | C46H46Cl2CuN10O10 | C40H36Cl2CuN8O11 | C20H34Cl2CuN4O12S2 |
| Formula weight | 785.08 | 1033.37 | 939.21 | 721.07 |
| Temperature/K | 301(2) | 301(3) | 301.2(8) | 299.6(6) |
| Crystal system | monoclinic | monoclinic | monoclinic | monoclinic |
| Space group | C2/c | P21/n | P2/n | I2/a |
| a/Å | 14.4924(10) | 10.2921(4) | 12.9264(5) | 17.9524(17) |
| b/Å | 11.1385(7) | 15.7544(4) | 11.1702(4) | 11.0913(7) |
| c/Å | 22.2237(13) | 15.3205(5) | 15.0564(10) | 16.3512(15) |
| α/° | 90 | 90 | 90 | 90 |
| β/° | 91.562(7) | 106.641(4) | 106.559(6) | 100.154(8) |
| γ/° | 90 | 90 | 90 | 90 |
| Volume/Å3 | 3586.1(4) | 2380.11(14) | 2083.83(19) | 3204.8(5) |
| Z | 4 | 2 | 2 | 4 |
| Goodness-of-fit on F2 | 1.117 | 1.024 | 1.081 | 1.035 |
| Final R indexes [I > = 2σ (I)] | R1 = 0.0575, wR2 = 0.1683 | R1 = 0.0338 wR2 = 0.0914 | R1 = 0.0473 wR2 = 0.1279 | R1 = 0.0340 wR2 = 0.0843 |
| Largest diff. peak/hole/e Å−3 | 0.60/−0.40 | 0.35/−0.25 | 0.62/−0.71 | 0.35/−0.32 |
| CCDC | 1942113 | 1942114 | 1942115 | 1942116 |
| Solvent. | CP | Coordinated Solvent | CP Dimensionality |
|---|---|---|---|
| DMSO 1 | 1 [17] | 2 DMSO | 3D |
| DMSO 2 | 2 [17] | 4 DMSO | 1D |
| DMA 1 | 3 | 2 H2O | 2D |
| DMF 1 | 43 | 2 DMF | 2D |
| DMF 1 | 54 | 2 H2O | 2D |
| DMSO/DMF 2 | 6 | 2 DMSO + 2 DMF | 1D |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rancan, M.; Carlotto, A.; Bottaro, G.; Armelao, L. Effect of Coordinating Solvents on the Structure of Cu(II)-4,4′-bipyridine Coordination Polymers. Inorganics 2019, 7, 103. https://doi.org/10.3390/inorganics7080103
Rancan M, Carlotto A, Bottaro G, Armelao L. Effect of Coordinating Solvents on the Structure of Cu(II)-4,4′-bipyridine Coordination Polymers. Inorganics. 2019; 7(8):103. https://doi.org/10.3390/inorganics7080103
Chicago/Turabian StyleRancan, Marzio, Alice Carlotto, Gregorio Bottaro, and Lidia Armelao. 2019. "Effect of Coordinating Solvents on the Structure of Cu(II)-4,4′-bipyridine Coordination Polymers" Inorganics 7, no. 8: 103. https://doi.org/10.3390/inorganics7080103
APA StyleRancan, M., Carlotto, A., Bottaro, G., & Armelao, L. (2019). Effect of Coordinating Solvents on the Structure of Cu(II)-4,4′-bipyridine Coordination Polymers. Inorganics, 7(8), 103. https://doi.org/10.3390/inorganics7080103