Synthesis and Evaluation of 99mTc-Tricabonyl Labeled Isonitrile Conjugates for Prostate-Specific Membrane Antigen (PSMA) Image
Abstract
:1. Introduction
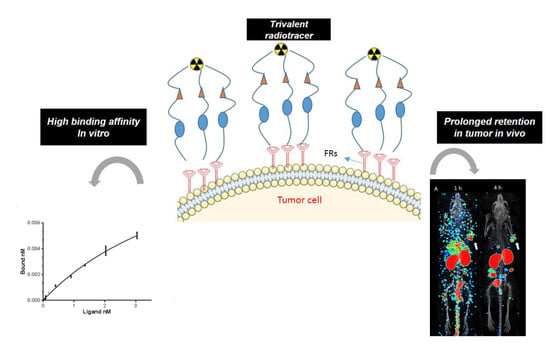
2. Results
2.1. Chemistry
2.2. Radiolabeling
2.3. In Vitro Serum Stability and Distribution Coefficient (LogD)
2.4. In Vitro Binding Affinity (Kd)
2.5. Ex Vivo Biodistribution
2.6. SPECT Imaging
3. Discussion
4. Experimental
4.1. General
4.2. Cell Culture and Tumor Model
4.3. Chemical Synthesis
4.4. Radiolabeling of [99mTc]Tc-15 and [99mTc]Tc-16
4.5. In Vitro Stability Tests in Serum
4.6. Determination of Distribution Coefficient (LogD Value)
4.7. Measurement of Binding Affinity (Kd) In Vitro
4.8. Ex Vivo Biodistribution Study
4.9. SPECT/CT Imaging
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA. Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Bouchelouche, K.; Choyke, P.L.; Capala, J. Prostate specific membrane antigen- a target for imaging and therapy with radionuclides. Discov. Med. 2010, 9, 55–61. [Google Scholar] [PubMed]
- Ghosh, A.; Heston, W.D. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J. Cell. Biochem. 2004, 91, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, J.; Hu, S.; He, S.; Bao, X.; Ma, G.; Luo, J.; Cheng, J.; Zhang, Y. 99mTc-labeling and evaluation of a HYNIC modified small-molecular inhibitor of prostate-specific membrane antigen. Nucl. Med. Biol. 2017, 48, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Perner, S.; Hofer, M.D.; Kim, R.; Shah, R.B.; Li, H.; Möller, P.; Hautmann, R.E.; Gschwend, J.E.; Kuefer, R.; Rubin, M.A. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum. Pathol. 2007, 38, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Bravaccini, S.; Puccetti, M.; Bocchini, M.; Ravaioli, S.; Celli, M.; Scarpi, E.; De Giorgi, U.; Tumedei, M.M.; Raulli, G.; Cardinale, L.; et al. PSMA expression: A potential ally for the pathologist in prostate cancer diagnosis. Sci. Rep. 2018, 8, 4254. [Google Scholar] [CrossRef] [Green Version]
- Wynant, G.E.; Murphy, G.P.; Horoszewicz, J.S.; Neal, C.E.; Collier, B.D.; Mitchell, E.; Purnell, G.; Tyson, I.; Heal, A.; Abdel-Nabi, H.; et al. Immunoscintigraphy of prostatic cancer: Preliminary results with 111In-labeled monoclonal antibody 7E11-C5.3 (CYT-356). Prostate 1991, 18, 229–241. [Google Scholar] [CrossRef]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.; Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar]
- Kahn, D.; Williams, R.D.; Manyak, M.J.; Haseman, M.K.; Seldin, D.W.; Libertino, J.A.; Maguire, R.T. Indium-Capromab Pendetide In the Evaluation of Patients with Residual or Recurrent Prostate Cancer After Radical Prostatectomy. J. Urol. 1998, 159, 2041–2047. [Google Scholar] [CrossRef]
- Nagda, S.N.; Mohideen, N.; Lo, S.S.; Khan, U.; Dillehay, G.; Wagner, R.; Campbell, S.; Flanigan, R. Long-term follow-up of 111In-capromab pendetide (ProstaScint) scan as pretreatment assessment in patients who undergo salvage radiotherapy for rising prostate-specific antigen after radical prostatectomy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 834–840. [Google Scholar] [CrossRef]
- Troyer, J.K.; Feng, Q.; Beckett, M.L.; Wright, G.L.J. Biochemical characterization and mapping of the 7E11-C5.3 epitope of the prostate-specific membrane antigen. Urol. Oncol. 1995, 1, 29–37. [Google Scholar] [CrossRef]
- Troyer, J.K.; Beckett, M.L.; Wright, G.L.J. Location of prostate-specific membrane antigen in the LNCaP prostate carcinoma cell line. Prostate 1997, 30, 232–242. [Google Scholar] [CrossRef]
- Bander, N.H.; Trabulsi, E.J.; Kostakoglu, L.; Yao, D.; Vallabhajosula, S.; Smith-Jones, P.; Joyce, M.A.; Milowsky, M.; Nanus, D.M.; Goldsmith, S.J. Targeting Metastatic Prostate Cancer with Radiolabeled Monoclonal Antibody J591 to the Extracellular Domain of Prostate Specific Membrane Antigen. J. Urol. 2003, 170, 1717–1721. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.J.; Divgi, C.R.; Pandit-Taskar, N.; Batraki, M.; Warren, N.; Nacca, A.; Smith-Jones, P.; Schwartz, L.; Kelly, W.K.; Slovin, S.; et al. Pilot Trial of Unlabeled and Indium-111–Labeled Anti–Prostate-Specific Membrane Antigen Antibody J591 for Castrate Metastatic Prostate Cancer. Clin. Cancer Res. 2005, 11, 7454–7461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bander, N.H.; Milowsky, M.I.; Nanus, D.M.; Kostakoglu, L.; Vallabhajosula, S.; Goldsmith, S.J. Phase I Trial of 177Lutetium-Labeled J591, a Monoclonal Antibody to Prostate-Specific Membrane Antigen, in Patients With Androgen-Independent Prostate Cancer. J. Clin. Oncol. 2005, 23, 4591–4601. [Google Scholar] [CrossRef]
- Pandit-Taskar, N.; O’Donoghue, J.A.; Morris, M.J.; Wills, E.A.; Schwartz, L.H.; Gonen, M.; Scher, H.I.; Larson, S.M.; Divgi, C.R. Antibody mass escalation study in patients with castration-resistant prostate cancer using 111In-J591: Lesion detectability and dosimetric projections for 90Y radioimmunotherapy. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2008, 49, 1066–1074. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, M.; Bauder-Wüst, U.; Leotta, K.; Zoller, F.; Mier, W.; Haberkorn, U.; Eisenhut, M.; Eder, M. A dimerized urea-based inhibitor of the prostate-specific membrane antigen for (68)Ga-PET imaging of prostate cancer. EJNMMI Res. 2012, 2, 23. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.R.; Foss, C.A.; Castanares, M.; Mease, R.C.; Byun, Y.; Fox, J.J.; Hilton, J.; Lupold, S.E.; Kozikowski, A.P.; Pomper, M.G. Synthesis and Evaluation of Technetium-99m- and Rhenium-Labeled Inhibitors of the Prostate-Specific Membrane Antigen (PSMA). J. Med. Chem. 2008, 51, 4504–4517. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Foss, C.A.; Byun, Y.; Nimmagadda, S.; Pullambhatla, M.; Fox, J.J.; Castanares, M.; Lupold, S.E.; Babich, J.W.; Mease, R.C.; et al. Radiohalogenated Prostate-Specific Membrane Antigen (PSMA)-Based Ureas as Imaging Agents for Prostate Cancer. J. Med. Chem. 2008, 51, 7933–7943. [Google Scholar] [CrossRef] [Green Version]
- Hillier, S.M.; Maresca, K.P.; Femia, F.J.; Marquis, J.C.; Foss, C.A.; Nguyen, N.; Zimmerman, C.N.; Barrett, J.A.; Eckelman, W.C.; Pomper, M.G.; et al. Preclinical Evaluation of Novel Glutamate-Urea-Lysine Analogues That Target Prostate-Specific Membrane Antigen as Molecular Imaging Pharmaceuticals for Prostate Cancer. Cancer Res. 2009, 69, 6932–6940. [Google Scholar] [CrossRef] [Green Version]
- Schottelius, M.; Wirtz, M.; Eiber, M.; Maurer, T.; Wester, H.-J. PSMA-I&T: Expanding the spectrum of PSMA-I&T applications towards SPECT and radioguided surgery. EJNMMI Res. 2015, 5, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maresca, K.P.; Marquis, J.C.; Hillier, S.M.; Lu, G.; Femia, F.J.; Zimmerman, C.N.; Eckelman, W.C.; Joyal, J.L.; Babich, J.W. Novel Polar Single Amino Acid Chelates for Technetium-99m Tricarbonyl-Based Radiopharmaceuticals with Enhanced Renal Clearance: Application to Octreotide. Bioconjug. Chem. 2010, 21, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.-H.; Hong, M.K.; Kim, Y.J.; Lee, Y.-S.; Lee, D.S.; Chung, J.-K.; Jeong, J.M. Development of a Ga-68 labeled PET tracer with short linker for prostate-specific membrane antigen (PSMA) targeting. Bioorg. Med. Chem. 2018, 26, 2501–2507. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.R.; Pullambhatla, M.; Byun, Y.; Nimmagadda, S.; Green, G.; Fox, J.J.; Horti, A.; Mease, R.C.; Pomper, M.G. 68Ga-labeled inhibitors of prostate-specific membrane antigen (PSMA) for imaging prostate cancer. J. Med. Chem. 2010, 53, 5333–5341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eder, M.; Schäfer, M.; Bauder-Wüst, U.; Hull, W.-E.; Wängler, C.; Mier, W.; Haberkorn, U.; Eisenhut, M. 68Ga-Complex Lipophilicity and the Targeting Property of a Urea-Based PSMA Inhibitor for PET Imaging. Bioconjug. Chem. 2012, 23, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.R.; Pullambhatla, M.; Foss, C.A.; Nimmagadda, S.; Ferdani, R.; Anderson, C.J.; Mease, R.C.; Pomper, M.G. 64Cu-Labeled Inhibitors of Prostate-Specific Membrane Antigen for PET Imaging of Prostate Cancer. J. Med. Chem. 2014, 57, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Hao, G.; Long, M.A.; Anthony, T.; Hsieh, J.T.; Sun, X. Imparting multivalency to a bifunctional chelator: A scaffold design for targeted PET imaging probes. Angew. Chem. Int. Ed. Engl. 2009, 48, 7346–7349. [Google Scholar] [CrossRef] [Green Version]
- Harada, N.; Kimura, H.; Onoe, S.; Watanabe, H.; Matsuoka, D.; Arimitsu, K.; Ono, M.; Saji, H. Synthesis and Biologic Evaluation of Novel 18F-Labeled Probes Targeting Prostate-Specific Membrane Antigen for PET of Prostate Cancer. J. Nucl. Med. 2016, 57, 1978–1984. [Google Scholar] [CrossRef] [Green Version]
- Ferro-Flores, G.; Luna-Gutiérrez, M.; Ocampo-García, B.; Santos-Cuevas, C.; Azorín-Vega, E.; Jiménez-Mancilla, N.; Orocio-Rodríguez, E.; Davanzo, J.; García-Pérez, F.O. Clinical translation of a PSMA inhibitor for 99mTc-based SPECT. Nucl. Med. Biol. 2017, 48, 36–44. [Google Scholar] [CrossRef]
- Vallabhajosula, S.; Nikolopoulou, A.; Babich, J.W.; Osborne, J.R.; Tagawa, S.T.; Lipai, I.; Solnes, L.; Maresca, K.P.; Armor, T.; Joyal, J.L.; et al. 99mTc-Labeled Small-Molecule Inhibitors of Prostate-Specific Membrane Antigen: Pharmacokinetics and Biodistribution Studies in Healthy Subjects and Patients with Metastatic Prostate Cancer. J. Nucl. Med. 2014, 55, 1791–1798. [Google Scholar] [CrossRef] [Green Version]
- Lutje, S.; Heskamp, S.; Cornelissen, A.S.; Poeppel, T.D.; van den Broek, S.A.; Rosenbaum-Krumme, S.; Bockisch, A.; Gotthardt, M.; Rijpkema, M.; Boerman, O.C. PSMA Ligands for Radionuclide Imaging and Therapy of Prostate Cancer: Clinical Status. Theranostics 2015, 5, 1388–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kratochwil, C.; Giesel, F.L.; Stefanova, M.; Benešová, M.; Bronzel, M.; Afshar-Oromieh, A.; Mier, W.; Eder, M.; Kopka, K.; Haberkorn, U. PSMA-Targeted Radionuclide Therapy of Metastatic Castration-Resistant Prostate Cancer with 177Lu-Labeled PSMA-617. J. Nucl. Med. 2016, 57, 1170–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillai, M.R.A.; Dash, A.; Knapp, F.F. Sustained Availability of 99mTc: Possible Paths Forward. J. Nucl. Med. 2013, 54, 313–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kularatne, S.A.; Zhou, Z.; Yang, J.; Post, C.B.; Low, P.S. Design, synthesis, and preclinical evaluation of prostate-specific membrane antigen targeted (99m)Tc-radioimaging agents. Mol. Pharm. 2009, 6, 790–800. [Google Scholar] [CrossRef]
- Lodhi, N.A.; Park, J.Y.; Kim, K.; Kim, Y.J.; Shin, J.H.; Lee, Y.-S.; Im, H.-J.; Jeong, J.M.; Khalid, M.; Cheon, G.J.; et al. Development of 99mTc-Labeled Human Serum Albumin with Prolonged Circulation by Chelate-then-Click Approach: A Potential Blood Pool Imaging Agent. Mol. Pharm. 2019. [Google Scholar] [CrossRef]
- Alberto, R.; Ortner, K.; Wheatley, N.; Schibli, R.; Schubiger, A.P. Synthesis and Properties of Boranocarbonate: A Convenient in Situ CO Source for the Aqueous Preparation of [99mTc(OH2)3(CO)3]+. J. Am. Chem. Soc. 2001, 123, 3135–3136. [Google Scholar] [CrossRef]
- Kasten, B.B.; Ma, X.; Liu, H.; Hayes, T.R.; Barnes, C.L.; Qi, S.; Cheng, K.; Bottorff, S.C.; Slocumb, W.S.; Wang, J.; et al. Clickable, Hydrophilic Ligand for fac-[MI(CO)3]+ (M = Re/99mTc) Applied in an S-Functionalized α-MSH Peptide. Bioconjug. Chem. 2014, 25, 579–592. [Google Scholar] [CrossRef]
- Mizuno, Y.; Uehara, T.; Hanaoka, H.; Endo, Y.; Jen, C.-W.; Arano, Y. Purification-Free Method for Preparing Technetium-99m-Labeled Multivalent Probes for Enhanced in Vivo Imaging of Saturable Systems. J. Med. Chem. 2016, 59, 3331–3339. [Google Scholar] [CrossRef]
- Lakić, M.; Sabo, L.; Ristić, S.; Savić, A.; Petričević, S.; Nikolić, N.; Vukadinović, A.; Janković, D.; Sabo, T.J.; Vranješ-Đurić, S. Synthesis and biological evaluation of 99mTc tricarbonyl complex of O,O′-diethylethylenediamine-N,N′-di-3-propanoate as potential tumour diagnostic agent. Appl. Organomet. Chem. 2016, 30, 81–88. [Google Scholar] [CrossRef]
- Abrams, M.J.; Davison, A.; Jones, A.G.; Costello, C.E.; Pang, H. Synthesis and characterization of hexakis(alkyl isocyanide) and hexakis(aryl isocyanide) complexes of technetium(I). Inorg. Chem. 1983, 22, 2798–2800. [Google Scholar] [CrossRef]
- Holman, B.L.; Jones, A.G.; Lister-James, J.; Davison, A.; Abrams, M.J.; Kirshenbaum, J.M.; Tumeh, S.S.; English, R.J. A new Tc-99m-labeled myocardial imaging agent, hexakis(t-butylisonitrile)-technetium(I) [Tc-99m TBI]: Initial experience in the human. J. Nucl. Med. 1984, 25, 1350–1355. [Google Scholar] [PubMed]
- Doroudi, A.; Saraji, F.; Erfani, M.; Saadati, S.; Kisast, A.; Ahmadi, F.; Etesami, B. The stability of 99mTc-MIBI (Sestamibi) complex samples which prepared under ultrasound irradiation technique versus boiling water bath method. J. Appl. Pharm. Sci. 2016, 6, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Hao, G.Y.; Zang, J.Y.; Zhu, L.; Guo, Y.Z.; Liu, B.L. Synthesis, separation and biodistribution of 99mTc-CO-MIBI complex. J. Label. Compd. Radiopharm. 2004, 47, 513–521. [Google Scholar] [CrossRef]
- Satpati, D.; Mallia, M.; Kothari, K.; Pillai, M.R.A. Comparative evaluation of [99mTc(H2O)3(CO)3]+ precursor synthesized by conventional method and by using carbonyl kit. J. Label. Compd. Radiopharm. 2004, 47, 657–668. [Google Scholar] [CrossRef]
- Hao, G.; Zang, J.; Liu, B. Preparation and biodistribution of novel 99mTc(CO)3-CNR complexes for myocardial imaging. J. Label. Compd. Radiopharm. 2007, 50, 13–18. [Google Scholar] [CrossRef]
- Duan, X.; Zhang, X.; Gan, Q.; Fang, S.A.; Ruan, Q.; Song, X.; Zhang, J. Novel 99mTc-labelled complexes with thymidine isocyanide: Radiosynthesis and evaluation as potential tumor imaging tracers. MedChemComm 2018, 9, 705–712. [Google Scholar] [CrossRef]
- Lodhi, N.A.; Park, J.Y.; Hong, M.K.; Kim, Y.J.; Lee, Y.S.; Cheon, G.J.; Jeong, J.M. Development of (99m)Tc-labeled trivalent isonitrile radiotracer for folate receptor imaging. Bioorg. Med. Chem. 2019. [Google Scholar] [CrossRef]
- Barinka, C.; Byun, Y.; Dusich, C.L.; Banerjee, S.R.; Chen, Y.; Castanares, M.; Kozikowski, A.P.; Mease, R.C.; Pomper, M.G.; Lubkowski, J. Interactions between Human Glutamate Carboxypeptidase II and Urea-Based Inhibitors: Structural Characterization. J. Med. Chem. 2008, 51, 7737–7743. [Google Scholar] [CrossRef] [Green Version]
- Kopka, K.; Benešová, M.; Bařinka, C.; Haberkorn, U.; Babich, J. Glu-Ureido–Based Inhibitors of Prostate-Specific Membrane Antigen: Lessons Learned During the Development of a Novel Class of Low-Molecular-Weight Theranostic Radiotracers. J. Nucl. Med. 2017, 58, 17S–26S. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Toriyabe, Y.; Kazak, M.; Berkman, C.E. Pseudoirreversible inhibition of prostate-specific membrane antigen by phosphoramidate peptidomimetics. Biochemistry 2008, 47, 12658–12660. [Google Scholar] [CrossRef]
- Benešová, M.; Schäfer, M.; Bauder-Wüst, U.; Afshar-Oromieh, A.; Kratochwil, C.; Mier, W.; Haberkorn, U.; Kopka, K.; Eder, M. Preclinical Evaluation of a Tailor-Made DOTA-Conjugated PSMA Inhibitor with Optimized Linker Moiety for Imaging and Endoradiotherapy of Prostate Cancer. J. Nucl. Med. 2015, 56, 914–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirtz, M.; Schmidt, A.; Schottelius, M.; Robu, S.; Gunther, T.; Schwaiger, M.; Wester, H.J. Synthesis and in vitro and in vivo evaluation of urea-based PSMA inhibitors with increased lipophilicity. EJNMMI Res. 2018, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Wurzer, A.; Pollmann, J.; Schmidt, A.; Reich, D.; Wester, H.-J.; Notni, J. Molar Activity of Ga-68 Labeled PSMA Inhibitor Conjugates Determines PET Imaging Results. Mol. Pharm. 2018, 15, 4296–4302. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-T.; Pan, J.; Zhang, Z.; Lau, J.; Merkens, H.; Zhang, C.; Colpo, N.; Lin, K.-S.; Bénard, F. Effects of Linker Modification on Tumor-to-Kidney Contrast of 68Ga-Labeled PSMA-Targeted Imaging Probes. Mol. Pharm. 2018, 15, 3502–3511. [Google Scholar] [CrossRef] [PubMed]
- Gorges, T.M.; Riethdorf, S.; von Ahsen, O.; Nastal, Y.P.; Rock, K.; Boede, M.; Peine, S.; Kuske, A.; Schmid, E.; Kneip, C.; et al. Heterogeneous PSMA expression on circulating tumor cells: A potential basis for stratification and monitoring of PSMA-directed therapies in prostate cancer. Oncotarget 2016, 7, 34930–34941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillier, S.M.; Kern, A.M.; Maresca, K.P.; Marquis, J.C.; Eckelman, W.C.; Joyal, J.L.; Babich, J.W. 123I-MIP-1072, a small-molecule inhibitor of prostate-specific membrane antigen, is effective at monitoring tumor response to taxane therapy. J. Nucl. Med. 2011, 52, 1087–1093. [Google Scholar] [CrossRef] [Green Version]
- Hillier, S.M.; Maresca, K.P.; Lu, G.; Merkin, R.D.; Marquis, J.C.; Zimmerman, C.N.; Eckelman, W.C.; Joyal, J.L.; Babich, J.W. 99mTc-Labeled Small-Molecule Inhibitors of Prostate-Specific Membrane Antigen for Molecular Imaging of Prostate Cancer. J. Nucl. Med. 2013, 54, 1369–1376. [Google Scholar] [CrossRef] [Green Version]
- Humblet, V.; Misra, P.; Bhushan, K.R.; Nasr, K.; Ko, Y.-S.; Tsukamoto, T.; Pannier, N.; Frangioni, J.V.; Maison, W. Multivalent scaffolds for affinity maturation of small molecule cell surface-binders and their application to prostate tumor targeting. J. Med. Chem. 2009, 52, 544–550. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Holt, G.E.; Velders, M.P.; Kwon, E.D.; Kast, W.M. Murine six-transmembrane epithelial antigen of the prostate, prostate stem cell antigen, and prostate-specific membrane antigen: Prostate-specific cell-surface antigens highly expressed in prostate cancer of transgenic adenocarcinoma mouse prostate mice. Cancer Res. 2001, 61, 5857–5860. [Google Scholar]
- Schmittgen, T.D.; Zakrajsek, B.A.; Hill, R.E.; Liu, Q.; Reeves, J.J.; Axford, P.D.; Singer, M.J.; Reed, M.W. Expression pattern of mouse homolog of prostate-specific membrane antigen (FOLH1) in the transgenic adenocarcinoma of the mouse prostate model. Prostate 2003, 55, 308–316. [Google Scholar] [CrossRef]
- Slusher, B.S.; Tsai, G.; Yoo, G.; Coyle, J.T. Immunocytochemical localization of the N-acetyl-aspartyl-glutamate (NAAG) hydrolyzing enzyme N-acetylated alpha-linked acidic dipeptidase (NAALADase). J. Comp. Neurol. 1992, 315, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Foss, C.A.; Mease, R.C.; Fan, H.; Wang, Y.; Ravert, H.T.; Dannals, R.F.; Olszewski, R.T.; Heston, W.D.; Kozikowski, A.P.; Pomper, M.G. Radiolabeled small-molecule ligands for prostate-specific membrane antigen: In vivo imaging in experimental models of prostate cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 4022–4028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacich, D.J.; Pinto, J.T.; Tong, W.P.; Heston, W.D. Cloning, expression, genomic localization, and enzymatic activities of the mouse homolog of prostate-specific membrane antigen/NAALADase/folate hydrolase. Mamm. Genome 2001, 12, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.; Amor-Coarasa, A.; Ponnala, S.; Nikolopoulou, A.; Williams, C.; Schlyer, D.; Zhao, Y.; Kim, D.; Babich, J.W. Trifunctional PSMA-targeting constructs for prostate cancer with unprecedented localization to LNCaP tumors. Eur. J. Nucl. Med. Mol. Imaging 2018. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.-Y.; Wu, H.; Lee, M.Y.; Xu, A.; Srinivasan, R.; Yao, S.Q. Solid-Phase Synthesis of Azidomethylene Inhibitors Targeting Cysteine Proteases. Org. Lett. 2008, 10, 1881–1884. [Google Scholar] [CrossRef]
- Skwarecki, A.S.; Skarbek, K.; Martynow, D.; Serocki, M.; Bylińska, I.; Milewska, M.J.; Milewski, S. Molecular Umbrellas Modulate the Selective Toxicity of Polyene Macrolide Antifungals. Bioconjug. Chem. 2018, 29, 1454–1465. [Google Scholar] [CrossRef]
- Lazarova, N.; James, S.; Babich, J.; Zubieta, J. A convenient synthesis, chemical characterization and reactivity of [Re(CO)3(H2O)3]Br: The crystal and molecular structure of [Re(CO)3(CH3CN)2Br]. Inorg. Chem. Commun. 2004, 7, 1023–1026. [Google Scholar] [CrossRef]







| Tissues | 1 h | 4 h | 1 h Blockade | 4 h Blockade |
|---|---|---|---|---|
| Blood | 0.60 ± 0.02 | 0.31 ± 0.02 | 0.52 ± 0.09 | 0.31 ± 0.01 |
| Muscle | 0.17 ± 0.07 | 0.08 ± 0.01 | 0.11 ± 0.02 | 0.08 ± 0.01 |
| Tumor | 1.48 ± 0.18 *** | 0.81 ± 0.09 *** | 0.38 ± 0.12 | 0.20 ± 0.04 |
| Heart | 0.22 ± 0.02 | 0.12 ± 0.01 | 0.16 ± 0.03 | 0.10 ± 0.01 |
| Lung | 0.64 ± 0.02 | 0.30 ± 0.04 | 0.61 ± 0.20 | 0.30 ± 0.05 |
| Liver | 0.94 ± 0.05 | 0.85 ± 0.05 | 0.84 ± 0.12 | 0.84 ± 0.03 |
| Spleen | 0.90 ± 0.14 | 0.26 ± 0.06 | 0.15 ± 0.03 | 0.16 ± 0.01 |
| Stomach | 0.31 ± 0.07 | 0.15 ± 0.02 | 0.17 ± 0.04 | 0.14 ± 0.01 |
| Intestine | 0.78 ± 0.07 | 0.81 ± 0.19 | 0.55 ± 0.13 | 0.97 ± 0.20 |
| Kidney | 59.59 ± 8.45 *** | 13.72 ± 5.45 ** | 4.22 ± 1.30 | 1.44 ± 009 |
| Bone | 0.34 ± 0.02 | 0.16 ± 0.03 | 0.26 ± 0.07 | 0.20 ± 0.03 |
| Tumor/blood | 2.50 ± 0.18 *** | 2.6 ± 0.13 *** | 0.73 ± 0.18 | 0.65 ± 0.13 |
| Tumor/muscle | 8.50 ± 2.80 ** | 10.4 ± 0.77 *** | 3.5 ± 0.50 | 2.71 ± 0.78 |
| Tumor/liver | 1.57 ± 0.15 ** | 0.96 ± 0.13 ** | 0.45 ± 0.10 | 0.24 ± 0.04 |
| Tumor/kidney | 0.02 ± 0.00 | 0.06 ± 0.02 | 0.09 ± 0.02 | 0.14 ± 0.03 |
| Tissues | 1 h | 4 h | 1 h Blockade | 4 h Blockade |
|---|---|---|---|---|
| Blood | 0.42 ± 0.01 | 0.17 ± 0.03 | 0.75 ± 0.08 | 0.15 ± 0.01 |
| Muscle | 0.13 ± 0.02 | 0.07 ± 0.01 | 0.22 ± 0.06 | 0.05 ± 0.01 |
| Tumor | 1.87 ± 0.11 *** | 2.83 ± 0.26 *** | 0.45 ± 0.02 | 0.39 ± 0.03 |
| Heart | 0.36 ± 0.01 | 0.25 ± 0.05 | 0.48 ± 0.05 | 0.11 ± 0.01 |
| Lung | 0.52 ± 0.05 | 0.36 ± 0.04 | 1.63 ± 0.10 | 0.45 ± 0.04 |
| Liver | 1.17 ± 0.04 | 0.69 ± 0.11 | 1.95 ± 0.07 | 1.33 ± 0.15 |
| Spleen | 3.40 ± 0.93 | 3.44 ± 0.23 | 0.40 ± 0.03 | 0.20 ± 0.04 |
| Stomach | 0.85 ± 0.23 | 0.42 ± 0.04 | 1.04 ± 0.26 | 0.23 ± 0.04 |
| Intestine | 0.47 ± 0.06 | 1.48 ± 0.18 | 0.85 ± 0.07 | 1.26 ± 0.14 |
| Kidney | 24.66 ± 2.17 ** | 39.65 ± 6.86 *** | 5.66 ± 0.63 | 3.59 ± 0.52 |
| Bone | 0.63 ± 0.03 | 1.04 ± 0.15 | 1.16 ± 0.08 | 0.47 ± 0.12 |
| Tumor/blood | 4.43 ± 0.39 *** | 16.70 ± 1.36 *** | 0.61 ± 0.07 | 2.67 ± 0.25 |
| Tumor/muscle | 14.05 ± 1.78 *** | 40.43 ± 3.97 *** | 2.16 ± 0.43 | 8.44 ± 1.52 |
| Tumor/liver | 1.60 ± 0.13 *** | 4.17 ± 0.36 *** | 0.23 ± 0.02 | 0.30 ± 0.04 |
| Tumor/kidney | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.11 ± 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lodhi, N.A.; Park, J.Y.; Kim, K.; Hong, M.K.; Kim, Y.J.; Lee, Y.-S.; Cheon, G.J.; Kang, K.W.; Jeong, J.M. Synthesis and Evaluation of 99mTc-Tricabonyl Labeled Isonitrile Conjugates for Prostate-Specific Membrane Antigen (PSMA) Image. Inorganics 2020, 8, 5. https://doi.org/10.3390/inorganics8010005
Lodhi NA, Park JY, Kim K, Hong MK, Kim YJ, Lee Y-S, Cheon GJ, Kang KW, Jeong JM. Synthesis and Evaluation of 99mTc-Tricabonyl Labeled Isonitrile Conjugates for Prostate-Specific Membrane Antigen (PSMA) Image. Inorganics. 2020; 8(1):5. https://doi.org/10.3390/inorganics8010005
Chicago/Turabian StyleLodhi, Nadeem Ahmed, Ji Yong Park, Kyuwan Kim, Mi Kyung Hong, Young Joo Kim, Yun-Sang Lee, Gi Jeong Cheon, Keon Wook Kang, and Jae Min Jeong. 2020. "Synthesis and Evaluation of 99mTc-Tricabonyl Labeled Isonitrile Conjugates for Prostate-Specific Membrane Antigen (PSMA) Image" Inorganics 8, no. 1: 5. https://doi.org/10.3390/inorganics8010005
APA StyleLodhi, N. A., Park, J. Y., Kim, K., Hong, M. K., Kim, Y. J., Lee, Y. -S., Cheon, G. J., Kang, K. W., & Jeong, J. M. (2020). Synthesis and Evaluation of 99mTc-Tricabonyl Labeled Isonitrile Conjugates for Prostate-Specific Membrane Antigen (PSMA) Image. Inorganics, 8(1), 5. https://doi.org/10.3390/inorganics8010005