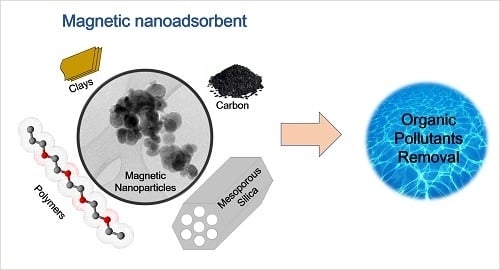
Nanomaterials with Tailored Magnetic Properties as Adsorbents of Organic Pollutants from Wastewaters
Abstract
:1. Introduction
2. Silica-Based Materials
3. Clay-Based Composites
4. Carbon-Based Materials
| Adsorbent | Surface Area (m2 g−1) | Magnetic Saturation (emu g−1) | Organic Pollutant | Adsorption Capacity (mg g−1) | Ref. |
|---|---|---|---|---|---|
| GO/Fe3O4 | 272 | - | 1-Naphthylamine 1-Naphthol Naphthalene | 2.85 2.70 2.63 | [76] |
| MWCNTs/Fe3O4 | 77 | - | 1-Naphthylamine 1-Naphthol Naphthalene | 1.45 1.13 1.05 | [76] |
| Magnetic-cyclodextrin–chitosan/graphene oxide | 402 | 55.1 | Methylene blue | 84.3 | [77] |
| Fe3O4-GS | - | 4.4 | Methylene blue | 526 | [81] |
| MWCNT | 61 | - | Methylene blue Neutral red Brilliant cresyl blue | 15.9 20.5 23.0 | [83] |
| Fe3O4/AC | 1200 | 16.5 | Rhodamine B Methyl orange | 182.4 150.3 | [86] |
| MWCNTs/Fe3O4/PANI | - | 42.9 | Methyl orange Congo red | 446.2 417.4 | [84] |
| Fe3O4/BC | 365 | 19.0 | Carbamazepine Tetracycline | 62.7 94.2 | [87] |
| Fe3O4/AC | 486 | 20.8 | Carbamazepine Tetracycline | 135.1 45.3 | [87] |
| Fe3O4-GOS | - | 1.1 | Tetracycline | 473 | [88] |
| CMNTs | 184 | 5.6 | Carbamazepine | 65 | [85] |
| MPAC | 430–780 | 5–30 | Diclofenac, Fluoxetine, Estradiol, Norethindrone, Atrazine, Carbamazepine, Deethylatrazine, Sulfamethoxazole and Caffeine | 1–80 | [89] |
| MCN | - | - | Metolachlor, Bisphenol A, Tonalide, Triclosan, Ketoprofen and Estriol | 18–28 | [82] |
5. Polymer-Based Materials
6. Waste-Based Materials
7. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- European Environment Agency. European Waters Assessment of Status and Pressures 2018; EEA Report; European Environment Agency: Luxembourg, 2018; ISBN 9789292139476. [Google Scholar]
- Stuart, M.; Lapworth, D.; Crane, E.; Hart, A. Review of risk from potential emerging contaminants in UK groundwater. Sci. Total Environ. 2012, 416, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bée, A.; Obeid, L.; Mbolantenaina, R.; Welschbillig, M.; Talbot, D. Magnetic chitosan/clay beads: A magsorbent for the removal of cationic dye from water. J. Magn. Magn. Mater. 2017, 421, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.; Ma, J.; Ma, Q.; Zhang, D.; Qiao, N.; Hu, M.; Ma, H. Adsorption of methylene blue onto Fe3O4/activated montmorillonite nanocomposite. Appl. Clay Sci. 2016, 119, 132–140. [Google Scholar] [CrossRef]
- Han, H.; Rafiq, M.K.; Zhou, T.; Xu, R.; Mašek, O.; Li, X. A critical review of clay-based composites with enhanced adsorption performance for metal and organic pollutants. J. Hazard. Mater. 2019, 369, 780–796. [Google Scholar] [CrossRef]
- Arya, V.; Philip, L. Adsorption of pharmaceuticals in water using Fe3O4 coated polymer clay composite. Microporous Mesoporous Mater. 2016, 232, 273–280. [Google Scholar] [CrossRef]
- Nagy, Z.M.; Molnár, M.; Fekete-Kertész, I.; Molnár-Perl, I.; Fenyvesi, É.; Gruiz, K. Removal of emerging micropollutants from water using cyclodextrin. Sci. Total Environ. 2014, 485, 711–719. [Google Scholar] [CrossRef]
- Yang, K.; Wu, W.; Jing, Q.; Zhu, L. Aqueous adsorption of aniline, phenol, and their substitutes by multi-walled carbon nanotubes. Environ. Sci. Technol. 2008, 42, 7931–7936. [Google Scholar] [CrossRef]
- Dailianis, S.; Tsarpali, V.; Melas, K.; Karapanagioti, H.K.; Manariotis, I.D. Aqueous phenanthrene toxicity after high-frequency ultrasound degradation. Aquat. Toxicol. 2014, 147, 32–40. [Google Scholar] [CrossRef]
- Magdy, A.; Fouad, Y.O.; Abdel-Aziz, M.H.; Konsowa, A.H. Synthesis and characterization of Fe3O4/kaolin magnetic nanocomposite and its application in wastewater treatment. J. Ind. Eng. Chem. 2017, 56, 299–311. [Google Scholar] [CrossRef]
- Diagboya, P.N.; Dikio, E.D. Scavenging of aqueous toxic organic and inorganic cations using novel facile magneto-carbon black-clay composite adsorbent. J. Clean. Prod. 2018, 180, 71–80. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Yun, Y.S. Spinel ferrite magnetic adsorbents: Alternative future materials for water purification? Coord. Chem. Rev. 2016, 315, 90–111. [Google Scholar] [CrossRef]
- Mehta, D.; Mazumdar, S.; Singh, S.K. Magnetic adsorbents for the treatment of water/wastewater—A review. J. Water Process Eng. 2015, 7, 244–265. [Google Scholar] [CrossRef]
- Gómez-Pastora, J.; Bringas, E.; Ortiz, I. Recent progress and future challenges on the use of high performance magnetic nano-adsorbents in environmental applications. Chem. Eng. J. 2014, 256, 187–204. [Google Scholar] [CrossRef]
- Larraza, I.; López-gónzalez, M.; Corrales, T.; Marcelo, G. Hybrid materials: Magnetite—Polyethylenimine—Montmorillonite, as magnetic adsorbents for Cr(VI) water treatment. J. Colloid Interface Sci. 2012, 385, 24–33. [Google Scholar] [CrossRef]
- Nisticò, R.; Cesano, F.; Garello, F. Magnetic Materials and Systems: Domain structure visualization and other characterization techniques for the application in the materials science and biomedicine. Inorganics 2020, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Nisticò, R. Magnetic materials and water treatments for a sustainable future. Res. Chem. Intermed. 2017, 43, 6911–6949. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, C.H.; Fiore, S.; Tong, D.S.; Zhang, H.; Li, C.S.; Ji, S.F.; Yu, W.H. Functional magnetic nanoparticle/clay mineral nanocomposites: Preparation, magnetism and versatile applications. Appl. Clay Sci. 2016, 127, 143–163. [Google Scholar] [CrossRef]
- Yuan, P.; Fan, M.; Yang, D.; He, H.; Liu, D.; Yuan, A.; Zhu, J.X.; Chen, T.H. Montmorillonite-supported magnetite nanoparticles for the removal of hexavalent chromium [Cr(VI)] from aqueous solutions. J. Hazard. Mater. 2009, 166, 821–829. [Google Scholar] [CrossRef]
- Mohammed, L.; Gomaa, H.G.; Ragab, D.; Zhu, J. Magnetic nanoparticles for environmental and biomedical applications: A review. Particuology 2017, 30, 1–14. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, S.Z.; Hu, Q.H.; Lu, G.Q. Magnetic nanocomposites with mesoporous structures: Synthesis and applications. Small 2011, 7, 425–443. [Google Scholar] [CrossRef]
- Tang, S.C.N.; Lo, I.M.C. Magnetic nanoparticles: Essential factors for sustainable environmental applications. Water Res. 2013, 47, 2613–2632. [Google Scholar] [CrossRef] [PubMed]
- Gao, F. An Overview of Surface-Functionalized Magnetic Nanoparticles: Preparation and Application for Wastewater Treatment. ChemistrySelect 2019, 4, 6805–6811. [Google Scholar] [CrossRef]
- Gómez-Pastora, J.; Dominguez, S.; Bringas, E.; Rivero, M.J.; Ortiz, I.; Dionysiou, D.D. Review and perspectives on the use of magnetic nanophotocatalysts (MNPCs) in water treatment. Chem. Eng. J. 2017, 310, 407–427. [Google Scholar] [CrossRef]
- Sharma, R.K.; Sharma, S.; Dutta, S.; Zboril, R.; Gawande, M.B. Silica-nanosphere-based organic-inorganic hybrid nanomaterials: Synthesis, functionalization and applications in catalysis. Green Chem. 2015, 17, 3207–3230. [Google Scholar] [CrossRef]
- Cashin, V.B.; Eldridge, D.S.; Yu, A.; Zhao, D. Surface functionalization and manipulation of mesoporous silica adsorbents for improved removal of pollutants: A review. Environ. Sci. Water Res. Technol. 2018, 4, 110–128. [Google Scholar] [CrossRef]
- Walcarius, A.; Mercier, L. Mesoporous organosilica adsorbents: Nanoengineered materials for removal of organic and inorganic pollutants. J. Mater. Chem. 2010, 20, 4478–4511. [Google Scholar] [CrossRef]
- Wan, Y.; Zhao, D. On the Controllable Soft-Templating Approach to Mesoporous Silicates. Chem. Rev. 2007, 107, 2821–2860. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Trewyn, B.G.; Lin, V.S.Y. Mesoporous silica nanoparticles: Structural design and applications. J. Mater. Chem. 2010, 20, 7924–7937. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Zhang, L.; Wang, P. Rational design of nanomaterials for water treatment. Nanoscale 2015, 7, 17167–17194. [Google Scholar] [CrossRef] [Green Version]
- Diagboya, P.N.E.; Dikio, E.D. Silica-based mesoporous materials; emerging designer adsorbents for aqueous pollutants removal and water treatment. Microporous Mesoporous Mater. 2018, 266, 252–267. [Google Scholar] [CrossRef]
- Cendrowski, K.; Sikora, P.; Zielinska, B.; Horszczaruk, E.; Mijowska, E. Chemical and thermal stability of core-shelled magnetite nanoparticles and solid silica. Appl. Surf. Sci. 2017, 407, 391–397. [Google Scholar] [CrossRef]
- Wang, P.; Wang, X.; Yu, S.; Zou, Y.; Wang, J.; Chen, Z.; Alharbi, N.S.; Alsaedi, A.; Hayat, T.; Chen, Y.; et al. Silica coated Fe3O4 magnetic nanospheres for high removal of organic pollutants from wastewater. Chem. Eng. J. 2016, 306, 280–288. [Google Scholar] [CrossRef]
- Wang, L.; Shen, C.; Cao, Y. PVP modified Fe3O4@SiO2 nanoparticles as a new adsorbent for hydrophobic substances. J. Phys. Chem. Solids 2019, 133, 28–34. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Q.; Liu, Y.; Lei, M. Recyclable nanoscale zero-valent iron-based magnetic polydopamine coated nanomaterials for the adsorption and removal of phenanthrene and anthracene. Sci. Technol. Adv. Mater. 2017, 18, 3–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, Q.; Zhang, Y.; Wang, C.; Wang, X.; Sun, Z.; Hou, X.F.; Zhao, D.; Deng, Y. Magnetic yolk-shell mesoporous silica microspheres with supported Au nanoparticles as recyclable high-performance nanocatalysts. J. Mater. Chem. A 2015, 3, 4586–4594. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, F.; Huang, D.; Hou, S. A facile route to magnetic mesoporous core–shell structured silicas containing covalently bound cyclodextrins for the removal of the antibiotic doxycycline from water. RSC Adv. 2018, 8, 31348–31357. [Google Scholar] [CrossRef] [Green Version]
- Oppmann, M.; Wozar, M.; Reichstein, J.; Mandel, K. Reusable Superparamagnetic Raspberry-Like Supraparticle Adsorbers as Instant Cleaning Agents for Ultrafast Dye Removal from Water. ChemNanoMat 2019, 5, 230–240. [Google Scholar] [CrossRef]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Fröba, M. Silica-based mesoporous organic–inorganic hybrid materials. Angew. Chem. Int. Ed. 2006, 45, 3216–3251. [Google Scholar] [CrossRef]
- Sasaki, T.; Tanaka, S. Adsorption behavior of some aromatic compounds on hydrophobic magnetite for magnetic separation. J. Hazard. Mater. 2011, 196, 327–334. [Google Scholar] [CrossRef]
- Chen, J.; Chen, H. Removal of anionic dyes from an aqueous solution by a magnetic cationic adsorbent modified with DMDAAC. New J. Chem. 2018, 42, 7262–7271. [Google Scholar] [CrossRef]
- Peralta, M.E.; Jadhav, S.A.; Magnacca, G.; Scalarone, D.; Mártire, D.O.; Parolo, M.E.; Carlos, L. Synthesis and in vitro testing of thermoresponsive polymer-grafted core–shell magnetic mesoporous silica nanoparticles for efficient controlled and targeted drug delivery. J. Colloid Interface Sci. 2019, 544, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Jiaqi, Z.; Yimin, D.; Danyang, L.; Shengyun, W.; Liling, Z.; Yi, Z. Synthesis of carboxyl-functionalized magnetic nanoparticle for the removal of methylene blue. Colloids Surf. A Physicochem. Eng. Asp. 2019, 572, 58–66. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, J.; Chen, X.; Yang, W.; Pei, H.; Hu, N.; Li, Z.; Suo, Y.; Li, T.; Wang, J. The simultaneous detection and removal of organophosphorus pesticides by a novel Zr-MOF based smart adsorbent. J. Mater. Chem. A 2018, 6, 2184–2192. [Google Scholar] [CrossRef]
- Ghorbani, F.; Kamari, S. Core–shell magnetic nanocomposite of Fe3O4@SiO2 @NH2 as an efficient and highly recyclable adsorbent of methyl red dye from aqueous environments. Environ. Technol. Innov. 2019, 14, 100333. [Google Scholar] [CrossRef]
- Peres, E.C.; Slaviero, J.C.; Cunha, A.M.; Hosseini-Bandegharaei, A.; Dotto, G.L. Microwave synthesis of silica nanoparticles and its application for methylene blue adsorption. J. Environ. Chem. Eng. 2018, 6, 649–659. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Ai, W.; Wei, C. A new method to prepare mesoporous silica from coal gasification fine slag and its application in methylene blue adsorption. J. Clean. Prod. 2019, 212, 1062–1071. [Google Scholar] [CrossRef]
- Brigante, M.; Parolo, M.E.; Schulz, P.C.; Avena, M. Synthesis, characterization of mesoporous silica powders and application to antibiotic remotion from aqueous solution. Effect of supported Fe-oxide on the SiO2 adsorption properties. Powder Technol. 2014, 253, 178–186. [Google Scholar] [CrossRef]
- Saikia, D.; Deka, J.R.; Wu, C.E.; Yang, Y.C.; Kao, H.M. pH responsive selective protein adsorption by carboxylic acid functionalized large pore mesoporous silica nanoparticles SBA-1. Mater. Sci. Eng. C 2019, 94, 344–356. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Bispo, C.; Bion, N.; Ferreira, P.; Batonneau-Gener, I. Periodic Mesoporous Organosilicas as adsorbents for the organic pollutants removal in aqueous phase. Microporous Mesoporous Mater. 2014, 200, 117–123. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, T.; Wang, S.; Niu, H.; Wang, X.; Cai, Y. One-pot synthesis of C18-functionalized core-shell magnetic mesoporous silica composite as efficient sorbent for organic dye. J. Colloid Interface Sci. 2015, 448, 189–196. [Google Scholar] [CrossRef]
- Huang, Y.; Fulton, A.N.; Keller, A.A. Optimization of porous structure of superparamagnetic nanoparticle adsorbents for higher and faster removal of emerging organic contaminants and PAHs. Environ. Sci. Water Res. Technol. 2016, 2, 521–528. [Google Scholar] [CrossRef] [Green Version]
- Ghanei, M.; Rashidi, A.; Tayebi, H.; Yazdanshenas, M.E. Removal of Acid Blue 25 from Aqueous Media by Magnetic-SBA-15/CPAA Super Adsorbent: Adsorption Isotherm, Kinetic, and Thermodynamic Studies. J. Chem. Eng. Data 2018, 63, 3592–3605. [Google Scholar] [CrossRef]
- Arica, T.A.; Ayas, E.; Arica, M.Y. Magnetic MCM-41 silica particles grafted with poly(glycidylmethacrylate) brush: Modification and application for removal of direct dyes. Microporous Mesoporous Mater. 2017, 243, 164–175. [Google Scholar] [CrossRef]
- Jin, C.; Wang, Y.; Tang, H.; Zhu, K.; Liu, X.; Wang, J. Versatile rattle-type magnetic mesoporous silica spheres, working as adsorbents and nanocatalyst containers. J. Sol-Gel Sci. Technol. 2016, 77, 279–287. [Google Scholar] [CrossRef]
- Wo, R.; Li, Q.L.; Zhu, C.; Zhang, Y.; Qiao, G.F.; Lei, K.Y.; Du, P.; Jiang, W. Preparation and Characterization of Functionalized Metal–Organic Frameworks with Core/Shell Magnetic Particles (Fe3O4@SiO2@MOFs) for Removal of Congo Red and Methylene Blue from Water Solution. J. Chem. Eng. Data 2019, 64, 2455–2463. [Google Scholar] [CrossRef]
- Kittappa, S.; Pichiah, S.; Kim, J.R.; Yoon, Y.; Snyder, S.A.; Jang, M. Magnetised nanocomposite mesoporous silica and its application for effective removal of methylene blue from aqueous solution. Sep. Purif. Technol. 2015, 153, 67–75. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent advances for dyes removal using novel adsorbents: A review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef]
- Musso, T.B.; Parolo, M.E.; Pettinari, G.; Francisca, F.M. Cu(II) and Zn(II) adsorption capacity of three different clay liner materials. J. Environ. Manag. 2014, 146, 50–58. [Google Scholar] [CrossRef]
- Betega de Paiva, L.; Rita, A.; Valenzuela, F.R. Organoclays: Properties, preparation and applications. Appl. Clay Sci. 2008, 42, 8–24. [Google Scholar] [CrossRef]
- Parolo, M.E.; Pettinari, G.R.; Musso, T.B.; Sánchez-Izquierdo, M.P.; Fernández, L.G. Characterization of organo-modified bentonite sorbents: The effect of modification conditions on adsorption performance. Appl. Surf. Sci. 2014, 320, 356–363. [Google Scholar] [CrossRef]
- Naranjo Pablo, M.; Sham Edgardo, L.; Rodriguez, C.E.; Torres Sánchez, R.M.; Monica, F. Identification and quantification of the interaction mechanisms between the cationic surfactant HDTMA-Br and montmorillonite. Clays Clay Miner. 2013, 61, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Roca Jalil, M.E.; Vieira, R.S.; Azevedo, D.; Baschini, M.; Sapag, K. Improvement in the adsorption of thiabendazole by using aluminum pillared clays. Appl. Clay Sci. 2013, 71, 55–63. [Google Scholar] [CrossRef]
- Guégan, R. Organoclay applications and limits in the environment. C. R. Chim. 2018, 22, 132–141. [Google Scholar] [CrossRef]
- Jaber, M.; Miehé-Brendlé, J. Organoclays. Preparation, Properties and Applications. In Ordered Porous Solids; Elsevier: Amsterdam, The Netherlands, 2009; pp. 31–49. [Google Scholar]
- Xu, X.; Chen, W.; Zong, S.; Ren, X.; Liu, D. Magnetic clay as catalyst applied to organics degradation in a combined adsorption and Fenton-like process. Chem. Eng. J. 2019, 373, 140–149. [Google Scholar] [CrossRef]
- Liu, H.; Chen, W.; Liu, C.; Liu, Y.; Dong, C. Magnetic mesoporous clay adsorbent: Preparation, characterization and adsorption capacity for atrazine. Microporous Mesoporous Mater. 2014, 194, 72–78. [Google Scholar] [CrossRef]
- Fizir, M.; Dramou, P.; Zhang, K.; Sun, C.; Pham-Huy, C.; He, H. Polymer grafted-magnetic halloysite nanotube for controlled and sustained release of cationic drug. J. Colloid Interface Sci. 2017, 505, 476–488. [Google Scholar] [CrossRef]
- Mu, B.; Tang, J.; Zhang, L.; Wang, A. Preparation, characterization and application on dye adsorption of a well-defined two-dimensional superparamagnetic clay/polyaniline/Fe3O4 nanocomposite. Appl. Clay Sci. 2016, 132, 7–16. [Google Scholar] [CrossRef]
- Khajeh, M.; Laurent, S.; Dastafkan, K. Nanoadsorbents: Classification, preparation, and applications (with emphasis on aqueous media). Chem. Rev. 2013, 113, 7728–7768. [Google Scholar] [CrossRef]
- Sherlala, A.I.A.; Raman, A.A.A.; Bello, M.M.; Asghar, A. A review of the applications of organo-functionalized magnetic graphene oxide nanocomposites for heavy metal adsorption. Chemosphere 2018, 193, 1004–1017. [Google Scholar] [CrossRef]
- Zhu, M.; Diao, G. Review on the progress in synthesis and application of magnetic carbon nanocomposites. Nanoscale 2011, 3, 2748–2767. [Google Scholar] [CrossRef]
- Boruah, P.K.; Sharma, B.; Hussain, N.; Das, M.R. Magnetically recoverable Fe3O4/graphene nanocomposite towards efficient removal of triazine pesticides from aqueous solution: Investigation of the adsorption phenomenon and specific ion effect. Chemosphere 2017, 168, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, J.; Mayer, P.; Jiang, G. Impacts of some environmentally relevant parameters on the sorption of polycyclic aromatic hydrocarbons to aqueous suspensions of fullerene. Environ. Toxicol. Chem. 2008, 27, 1868–1874. [Google Scholar] [CrossRef]
- Yang, K.; Wang, X.; Zhu, L.; Xing, B. Competitive sorption of pyrene, phenanthrene, and naphthalene on multiwalled carbon nanotubes. Environ. Sci. Technol. 2006, 40, 5804–5810. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, J.; Wen, T.; Ren, X.; Huang, Y.; Wang, X. Colloids and Surfaces A: Physicochemical and Engineering Aspects Adsorption of naphthalene and its derivatives on magnetic graphene composites and the mechanism investigation. Colloids Surf. A Physicochem. Eng. Asp. 2013, 422, 118–125. [Google Scholar] [CrossRef]
- Fan, L.; Luo, C.; Sun, M.; Qiu, H.; Li, X. Synthesis of magnetic β-cyclodextrin-chitosan/graphene oxide as nanoadsorbent and its application in dye adsorption and removal. Colloids Surf. B Biointerfaces 2013, 103, 601–607. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, C.; Chen, Z. Removal of methylene blue from aqueous solution by a solvothermal-synthesized graphene/magnetite composite. J. Hazard. Mater. 2011, 192, 1515–1524. [Google Scholar] [CrossRef]
- Eom, D.; Prezzi, D.; Rim, K.T.; Zhou, H.; Lefenfeld, M.; Xiao, S.; Nuckolls, C.; Hybertsen, M.S.; Heinz, T.F.; Flynn, G.W. Structure and electronic properties of graphene nanoislands on CO(0001). Nano Lett. 2009, 9, 2844–2848. [Google Scholar] [CrossRef]
- Ramesha, G.K.; Vijaya Kumara, A.; Muralidhara, H.B.; Sampath, S. Graphene and graphene oxide as effective adsorbents toward anionic and cationic dyes. J. Colloid Interface Sci. 2011, 361, 270–277. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, X.; Xie, J.; Wu, R.; Liu, X.; Li, H.; Chen, F.; Yang, H.; Ming, Z.; Yang, S.-T. Magnetic graphene sponge for the removal of methylene blue. Appl. Surf. Sci. 2015, 351, 765–771. [Google Scholar] [CrossRef]
- Alizadeh Fard, M.; Barkdoll, B. Using recyclable magnetic carbon nanotube to remove micropollutants from aqueous solutions. J. Mol. Liq. 2018, 249, 193–202. [Google Scholar] [CrossRef]
- Gong, J.L.; Wang, B.; Zeng, G.M.; Yang, C.P.; Niu, C.G.; Niu, Q.Y.; Zhou, W.J.; Liang, Y. Removal of cationic dyes from aqueous solution using magnetic multi-wall carbon nanotube nanocomposite as adsorbent. J. Hazard. Mater. 2009, 164, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, H.; Li, J.; Chen, C. Hierarchical MWCNTs/Fe3O4/PANI magnetic composite as adsorbent for methyl orange removal. J. Colloid Interface Sci. 2015, 450, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Ok, Y.S.; Mohan, D.; Pittman, C.U.; Dou, X. Carbamazepine removal from water by carbon dot-modified magnetic carbon nanotubes. Environ. Res. 2019, 169, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tian, J.; Li, Y.; Sun, N.; Mi, S.; Xie, Y.; Chen, Z. Enhanced dyes adsorption from wastewater via Fe3O4 nanoparticles functionalized activated carbon. J. Hazard. Mater. 2019, 373, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Deng, S.; Zhao, T.; Wang, B.; Wang, Y.; Huang, J.; Yu, G.; Winglee, J.; Wiesner, M.R. Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling. J. Hazard. Mater. 2016, 305, 156–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, B.; Bai, Y.; Ming, Z.; Yang, H.; Chen, L.; Hu, X.; Feng, S.; Yang, S.T. Adsorption behaviors of tetracycline on magnetic graphene oxide sponge. Mater. Chem. Phys. 2017, 198, 283–290. [Google Scholar] [CrossRef]
- Lompe, K.M.; Vo Duy, S.; Peldszus, S.; Sauvé, S.; Barbeau, B. Removal of micropollutants by fresh and colonized magnetic powdered activated carbon. J. Hazard. Mater. 2018, 360, 349–355. [Google Scholar] [CrossRef]
- Alaba, P.A.; Oladoja, N.A.; Sani, Y.M.; Ayodele, O.B.; Mohammed, I.Y.; Olupinla, S.F.; Daud, W.M.W. Insight into wastewater decontamination using polymeric adsorbents. J. Environ. Chem. Eng. 2018, 6, 1651–1672. [Google Scholar] [CrossRef]
- Lv, S.W.; Liu, J.M.; Wang, Z.H.; Ma, H.; Li, C.Y.; Zhao, N.; Wang, S. Recent advances on porous organic frameworks for the adsorptive removal of hazardous materials. J. Environ. Sci. 2019, 80, 169–185. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, Y.; Xiao, J.; Fan, H.; Chen, C. Preparation and characterization of magnetic nanomaterial and its application for removal of polycyclic aromatic hydrocarbons. J. Hazard. Mater. 2019, 371, 323–331. [Google Scholar] [CrossRef]
- Huo, Y.; Wu, H.; Wang, Z.; Wang, F.; Liu, Y.; Feng, Y.; Zhao, Y. Preparation of core/shell nanocomposite adsorbents based on amine polymer-modified magnetic materials for the efficient adsorption of anionic dyes. Colloids Surf. A Physicochem. Eng. Asp. 2018, 549, 174–183. [Google Scholar] [CrossRef]
- Liu, D.; Huang, Z.; Li, M.; Sun, P.; Yu, T.; Zhou, L. Novel porous magnetic nanospheres functionalized by β-cyclodextrin polymer and its application in organic pollutants from aqueous solution. Environ. Pollut. 2019, 250, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Budd, P.M.; Ghanem, B.S.; Makhseed, S.; McKeown, N.B.; Msayib, K.J.; Tattershall, C.E. Polymers of intrinsic microporosity (PIMs): Robust, solution-processable, organic nanoporous materials. Chem. Commun. 2004, 4, 230–231. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Bansal, V.; Kim, K.H.; Kwon, E.E. Metal–organic frameworks (MOFs) as futuristic options for wastewater treatment. J. Ind. Eng. Chem. 2018, 62, 130–145. [Google Scholar] [CrossRef]
- Han, S.S.; Furukawa, H.; Yaghi, O.M.; Iii, W.A.G. Covalent organic frameworks as exceptional hydrogen storage materials. J. Am. Chem. Soc. 2008, 105, 11580–11581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben, T.; Ren, H.; Shengqian, M.; Cao, D.; Lan, J.; Jing, X.; Wang, W.; Xu, J.; Deng, F.; Simmons, J.M.; et al. Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area. Angew. Chem. Int. Ed. 2009, 48, 9457–9460. [Google Scholar] [CrossRef]
- Germain, J.; Hradil, J.; Fréchet, J.M.J.; Svec, F. High surface area nanoporous polymers for reversible hydrogen storage. Chem. Mater. 2006, 18, 4430–4435. [Google Scholar] [CrossRef]
- Hu, A.; Yang, X.; You, Q.; Liu, Y.; Wang, Q.; Liao, G.; Wang, D. Magnetically hyper-cross-linked polymers with well-developed mesoporous: A broad-spectrum and highly efficient adsorbent for water purification. J. Mater. Sci. 2019, 54, 2712–2728. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Ai, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Combined experimental and theoretical investigation on selective removal of mercury ions by metal organic frameworks modified with thiol groups. Chem. Eng. J. 2018, 354, 790–801. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Keser Demir, N.; Chen, J.P.; Li, K. Applications of water stable metal-organic frameworks. Chem. Soc. Rev. 2016, 45, 5107–5134. [Google Scholar] [CrossRef]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A review on metal–organic frameworks: Synthesis and applications. TrAC Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Yang, Q.; Ren, S.S.; Zhao, Q.; Lu, R.; Hang, C.; Chen, Z.; Zheng, H. Selective separation of methyl orange from water using magnetic ZIF-67 composites. Chem. Eng. J. 2018, 333, 49–57. [Google Scholar] [CrossRef]
- Hamedi, A.; Zarandi, M.B.; Nateghi, M.R. Highly efficient removal of dye pollutants by MIL-101(Fe) metal–organic framework loaded magnetic particles mediated by Poly L-Dopa. J. Environ. Chem. Eng. 2019, 7, 102882. [Google Scholar] [CrossRef]
- Wu, G.; Ma, J.; Li, S.; Guan, J.; Jiang, B.; Wang, L.; Li, J.; Wang, X.; Chen, L. Magnetic copper-based metal organic framework as an effective and recyclable adsorbent for removal of two fluoroquinolone antibiotics from aqueous solutions. J. Colloid Interface Sci. 2018, 528, 360–371. [Google Scholar] [CrossRef]
- Han, S.S.; Mendoza-Cortés, J.L.; Goddard, W.A. Recent advances on simulation and theory of hydrogen storage in metal-organic frameworks and covalent organic frameworks. Chem. Soc. Rev. 2009, 38, 1460–1476. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, S.P.S.; Romero, V.; Espiña, B.; Salonen, L.M. Tailoring Covalent Organic Frameworks to Capture Water Contaminants. Chem. A Eur. J. 2019, 25, 6461–6473. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Chen, Y.; Huang, L.; Lin, Z.; Cai, Z. Core-Shell Structured Magnetic Covalent Organic Framework Nanocomposites for Triclosan and Triclocarban Adsorption. ACS Appl. Mater. Interfaces 2019, 11, 22492–22500. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.X.; Yan, X.P. Controllable preparation of core–shell magnetic covalent-organic framework nanospheres for efficient adsorption and removal of bisphenols in aqueous solution. Chem. Commun. 2017, 53, 2511–2514. [Google Scholar] [CrossRef]
- He, S.; Zeng, T.; Wang, S.; Niu, H.; Cai, Y. Facile synthesis of magnetic covalent organic framework with three-dimensional bouquet-like structure for enhanced extraction of organic targets. ACS Appl. Mater. Interfaces 2017, 9, 2959–2965. [Google Scholar] [CrossRef]
- Wang, B.; Wan, Y.; Zheng, Y.; Lee, X.; Liu, T.; Yu, Z.; Huang, J.; Ok, Y.S.; Chen, J.; Gao, B. Alginate-based composites for environmental applications: A critical review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 318–356. [Google Scholar] [CrossRef]
- Lee, K.Y.; Rowley, J.A.; Eiselt, P.; Moy, E.M.; Bouhadir, K.H.; Mooney, D.J. Controlling mechanical and swelling properties of alginate hydrogels independently by cross-linker type and cross-linking density. Macromolecules 2000, 33, 4291–4294. [Google Scholar] [CrossRef]
- Urquiza, T.K.V.; Pérez, O.P.; Saldaña, M.G. Effect of the cross-linking with calcium ions on the structural and thermo-mechanical properties of alginate films. Mater. Res. Soc. Symp. Proc. 2011, 1355, 16–21. [Google Scholar]
- Talbot, D.; Abramson, S.; Griffete, N.; Bée, A. pH-sensitive magnetic alginate/γ-Fe2O3 nanoparticles for adsorption/desorption of a cationic dye from water. J. Water Process Eng. 2018, 25, 301–308. [Google Scholar] [CrossRef]
- Mohammadi, A.; Daemi, H.; Barikani, M. Fast removal of malachite green dye using novel superparamagnetic sodium alginate-coated Fe3O4 nanoparticles. Int. J. Biol. Macromol. 2014, 69, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Shen, C.; Ni, Y.; Tong, S.; Yu, F. Glow discharge plasma in water: A green approach to enhancing ability of chitosan for dye removal. J. Hazard. Mater. 2012, 201, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Li, T.T.; Liu, Y.G.; Peng, Q.Q.; Hu, X.J.; Liao, T.; Wang, H.; Lu, M. Removal of lead(II) from aqueous solution with ethylenediamine-modified yeast biomass coated with magnetic chitosan microparticles: Kinetic and equilibrium modeling. Chem. Eng. J. 2013, 214, 189–197. [Google Scholar] [CrossRef]
- Park, S.I.; Kwak, I.S.; Won, S.W.; Yun, Y.S. Glutaraldehyde-crosslinked chitosan beads for sorptive separation of Au(III) and Pd(II): Opening a way to design reduction-coupled selectivity-tunable sorbents for separation of precious metals. J. Hazard. Mater. 2013, 248, 211–218. [Google Scholar] [CrossRef]
- Tirtom, V.N.; Dinçer, A.; Becerik, S.; Aydemir, T.; Çelik, A. Comparative adsorption of Ni(II) and Cd(II) ions on epichlorohydrin crosslinked chitosan-clay composite beads in aqueous solution. Chem. Eng. J. 2012, 197, 379–386. [Google Scholar] [CrossRef]
- Zheng, C.; Zheng, H.; Wang, Y.; Sun, Y.; An, Y.; Liu, H.; Liu, S. Modified magnetic chitosan microparticles as novel superior adsorbents with huge “force field” for capturing food dyes. J. Hazard. Mater. 2019, 367, 492–503. [Google Scholar] [CrossRef]
- Xu, B.; Zheng, C.; Zheng, H.; Wang, Y.; Zhao, C.; Zhao, C.; Zhang, S. Polymer-grafted magnetic microspheres for enhanced removal of methylene blue from aqueous solutions. RSC Adv. 2017, 7, 47029–47037. [Google Scholar] [CrossRef] [Green Version]
- Ali, I.; Peng, C.; Naz, I.; Lin, D.; Saroj, D.P.; Ali, M. Development and application of novel bio-magnetic membrane capsules for the removal of the cationic dye malachite green in wastewater treatment. RSC Adv. 2019, 9, 3625–3646. [Google Scholar] [CrossRef] [Green Version]
- Jodeh, S.; Hamed, O.; Melhem, A.; Salghi, R.; Jodeh, D.; Azzaoui, K.; Benmassaoud, Y.; Murtada, K. Magnetic nanocellulose from olive industry solid waste for the effective removal of methylene blue from wastewater. Environ. Sci. Pollut. Res. 2018, 25, 22060–22074. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.X.; Omer, A.M.; Hu, Z.H.; Wang, Y.G.; Yu, D.; Ouyang, X.K. Efficient adsorption of diclofenac sodium from aqueous solutions using magnetic amine-functionalized chitosan. Chemosphere 2019, 217, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, H.; Zhang, W.; Yang, X.; Zhou, H.; Pan, Z.; Wang, D. Preparation of magnetic polyimide@ Mg-Fe layered double hydroxides core-shell composite for effective removal of various organic contaminants from aqueous solution. Chemosphere 2019, 219, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Ahamad, T.; Naushad, M.; Alshehri, S.M. International Journal of Biological Macromolecules Ultra-fast spill oil recovery using a mesoporous lignin based nanocomposite prepared from date palm pits (Phoenix dactylifera L.). Int. J. Biol. Macromol. 2019, 130, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.P.; Jaria, G.; Otero, M.; Esteves, V.I.; Calisto, V. Waste-based alternative adsorbents for the remediation of pharmaceutical contaminated waters: Has a step forward already been taken? Bioresour. Technol. 2018, 250, 888–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anastopoulos, I.; Bhatnagar, A.; Hameed, B.H.; Ok, Y.S.; Omirou, M. A review on waste-derived adsorbents from sugar industry for pollutant removal in water and wastewater. J. Mol. Liq. 2017, 240, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Sayehi, M.; Tounsi, H.; Garbarino, G.; Riani, P.; Busca, G. Reutilization of silicon- and aluminum- containing wastes in the perspective of the preparation of SiO2-Al2O3 based porous materials for adsorbents and catalysts. Waste Manag. 2020, 103, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Carrott, P.J.M.; Ribeiro Carrott, M.M.L. Suhas Low-Cost adsorbents: Growing approach to wastewater treatmenta review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 783–842. [Google Scholar] [CrossRef]
- Safarik, I.; Lunackova, P.; Weyda, F.; Safarikova, M. Adsorption of water-soluble organic dyes on ferrofluid- modified sawdust. Holzforschung 2007, 61, 247–253. [Google Scholar] [CrossRef]
- Safarik, I.; Safarikova, M. Magnetic fluid modified peanut husks as an adsorbent for organic dyes removal. Phys. Procedia 2010, 9, 274–278. [Google Scholar] [CrossRef] [Green Version]
- Zuorro, A.; Di Battista, A.; Lavecchia, R. Magnetically modified coffee silverskin for the removal of xenobiotics from wastewater. Chem. Eng. Trans. 2013, 35, 1375–1380. [Google Scholar]
- Minh, T.P.; Lebedeva, O.E. Adsorption Properties of a Magnetite Composite with Coffee Waste. Russ. J. Phys. Chem. A 2018, 92, 2044–2047. [Google Scholar] [CrossRef]
- Stan, M.; Lung, I.; Soran, M.; Opris, O.; Leostean, C.; Popa, A.; Copaciu, F.; Diana, M.; Kacso, I.; Silipas, T.; et al. Starch-coated green synthesized magnetite nanoparticles for removal of textile dye Optilan Blue from aqueous media. J. Taiwan Inst. Chem. Eng. 2019, 100, 65–73. [Google Scholar] [CrossRef]
- Aydin, S. Removal of Organophosphorus Pesticides from Aqueous Solution by Magnetic Fe3O4/Red Mud- Nanoparticles. Water Environ. Res. 2016, 88, 2275–2284. [Google Scholar] [CrossRef]
- Aydin, S.; Aydin, M.E.; Beduk, F.; Ulvi, A. Removal of antibiotics from aqueous solution by using magnetic Fe3O4/red mud-nanoparticles. Sci. Total Environ. 2019, 670, 539–546. [Google Scholar] [CrossRef]
- Madrakian, T.; Afkhami, A.; Ahmadi, M. Adsorption and kinetic studies of seven different organic dyes onto magnetite nanoparticles loaded tea waste and removal of them from wastewater samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 99, 102–109. [Google Scholar] [CrossRef]
- Madrakian, E.; Ghaemi, E.; Ahmadi, M. Magnetic Solid Phase Extraction and Removal of Five Cationic Dyes from Aqueous Solution Using Magnetite Nanoparticle Loaded Platanusorientalis Waste Leaves. Anal. Bioanal. Chem. Res. 2016, 3, 279–286. [Google Scholar]
- Sun, L.; Yuan, D.; Wan, S.; Yu, Z.; Dang, J. Adsorption Performance and Mechanisms of Methylene Blue Removal by Non-magnetic and Magnetic Particles Derived from the Vallisneria natans Waste. J. Polym. Environ. 2018, 26, 2992–3004. [Google Scholar] [CrossRef]
- Yu, J.X.; Wang, L.Y.; Chi, R.A.; Zhang, Y.F.; Xu, Z.G.; Guo, J. A simple method to prepare magnetic modified beer yeast and its application for cationic dye adsorption. Environ. Sci. Pollut. Res. 2013, 20, 543–551. [Google Scholar] [CrossRef]
- Magnacca, G.; Allera, A.; Montoneri, E.; Celi, L.; Benito, D.E.; Gagliardi, L.G.; Carlos, L. Novel magnetite nanoparticles coated with waste sourced bio- based substances as sustainable and renewable adsorbing materials. ACS Sustain. Chem. Eng. 2014, 2, 1518–1524. [Google Scholar] [CrossRef] [Green Version]
- Nisticò, R.; Cesano, F.; Franzoso, F.; Magnacca, G.; Scarano, D.; Funes, I.G.; Carlos, L.; Parolo, M.E. From biowaste to magnet-responsive materials for water remediation from polycyclic aromatic hydrocarbons. Chemosphere 2018, 202, 686–693. [Google Scholar] [CrossRef]
- Nisticò, R.; Celi, L.R.; Bianco Prevot, A.; Carlos, L.; Magnacca, G.; Zanzo, E.; Martin, M. Sustainable magnet-responsive nanomaterials for the removal of arsenic from contaminated water. J. Hazard. Mater. 2018, 342, 260–269. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Y.; Zhu, S.; Yang, J.; Song, J.; Fan, W.; Yu, H.; Bian, D.; Huo, M. Synthesis and characterization of a magnetic adsorbent from negatively-valued iron mud for methylene blue adsorption. PLoS ONE 2018, 13, e0191229. [Google Scholar] [CrossRef] [Green Version]


| Adsorbent | Surface Area (m2 g−1) | Magnetic Saturation (emu g−1) | Organic Pollutant | Adsorption Capacity (mg g−1) | Ref. |
|---|---|---|---|---|---|
| Fe3O4@SiO2 | - | 48.06 | Congo red | 50.54 | [33] |
| Fe3O4@SiO2-PVP | 60.82 | 30.89 | Phenanthrene | 18.84 | [34] |
| Fe3O4@SiO2–VTEOS–DMDAAC | - | - | Methylene blue | 109.89 | [41] |
| Fe3O4@SiO2-EDA-COOH | - | 58.7 | Methylene blue | 43.15 | [43] |
| Fe3O4@SiO2@Zn−TDPAT | - | >20 | Methylene blue Congo red | 58.67 17.73 | [56] |
| Fe3O4@SiO2@UiO-67 | - | 20.9 | Glyphosate | 256.54 | [44] |
| Fe@SiO2@PDA | - | 51.98 | Anthracene Phenanthrene | 0.484 0.184 | [35] |
| Fe3O4/SiO2 10 nm SP | 193 | >25 | Methylene blue | 93 | [38] |
| Fe3O4@SiO2-NH2 | - | >40 | Methylene red | 81.39 | [45] |
| Fe3O4@SiO2@mSiO2-CD | 119 | 30.99 | Doxycycline | 78 | [37] |
| Fe3O4@SiO2-C18 | 303 | 22.62 | Methylene blue | 363.64 | [51] |
| γ-Fe2O3@mSiO2-TPODAC | 1.63 | 7.09 | Methyl orange Gemfibrozil Sulfamethoxazole Acenaphtene Phenanthrene | 104 50 50 0.83 0.95 | [52] |
| mMCM-41-g-p(GMA)-TAEA | 185 | 19.6 | Direct blue-6 Direct black-38 | 142.7 79.9 | [54] |
| M-SBA-15/CPAA | 159 | 2.68 | Acid blue 25 | 909.09 | [53] |
| γ-Fe2O3@SiO2@h-mSiO2 | 329 | - | Methylene blue | 41 | [55] |
| MNCM-1 | 576 | 2.9 | Methylene blue | 248 | [57] |
| Adsorbent | Surface Area (m2 g−1) | Magnetic Saturation (emu g−1) | Organic Pollutant | Adsorption Capacity (mg g−1) | Ref. |
|---|---|---|---|---|---|
| Clay:chitosan:PAC:MNP | 95 | 1.91 | Atenolol | 15.6 | [6] |
| Ciprofloxacin | 39.1 | ||||
| Gemfibrozil | 24.8 | ||||
| Fe3O4/Mt | 148 | - | Methylene blue | 106.4 | [4] |
| Magnetic chitosan/clay beads | - | - | Methylene blue | 82 | [3] |
| MSEP | 112 | 31.8 | Atrazine | 1.79 | [67] |
| MHNTs | - | 42.87 | Norfloxacin | 99.6 | [68] |
| Fe3O4/kaolin | 32 | 12.32 | Direct red 23 | 22.88 | [10] |
| Fe3O4-Sep | 81 | 26.22 | Bisphenol A | - | [66] |
| BMF-1 | - | - | Methylene blue | 14.93 | [11] |
| BMF-0.5 | - | - | Methylene blue | 12.35 | [11] |
| Mt/PANI/Fe3O4-2 | - | 36.52 | Methylene blue | 184.5 | [69] |
| Adsorbent | Surface Area (m2 g−1) | Magnetic Saturation (emu g−1) | Organic Pollutant | Adsorption Capacity (mg g−1) | Ref. |
|---|---|---|---|---|---|
| Bio-magnetic membrane capsules from PVA–alginate matrix | - | 11.02 | Malachite green | 500 | [123] |
| Magnetic nanocellulose from olive industry solid waste | - | 21.4 | Methylene blue | 166.67 | [124] |
| Fe3O4-amine-functionalized chitosan with p-Benzoquinone | - | 17.5 | Diclofenac sodium | 469.48 | [125] |
| Magnetic β-cyclodextrin porous polymer nanospheres | 70.63 | 44.8 | Methylene blue | 305.8 | [94] |
| Magnetic porphyrin-based porous organic polymer | 310 | 45.9 | Phenylurea herbicides | Metoxuron = 1.13, Mono-linuron = 0.95, Chlorotoluron = 0.86, Buturon = 1.10 | [92] |
| Magnetic copper based metal–organic frameworks (MOF) | 327.9 | 44 | Fluoroquinolone antibiotics | Ciprofloxacin= 538 Norfloxacin =513 | [106] |
| Magnetic polyimide-Mg-Fe layered double hydroxides core–shell composite | 26.38 | Tetracycline 2,4-dichlorophenol Glyphosate | 185.53 176.06 190.84 | [126] | |
| Magnetic mesoporous lignin from date palm pits | 640 | 37.81 | Diesel Gasoline | Diesel = 22370 Gasoil = 21010 | [127] |
| Adsorbent | Surface Area (m g−1) | Magnetic Saturation (emu g−1) | Organic Pollutant | Adsorption Capacity (mg g−1) | Ref. |
|---|---|---|---|---|---|
| Magnetic Sawdust | - | - | Crystal violet | 51.2 | [132] |
| Magnetic Peanut husk | - | - | Acridine orange Bismark brown Crystal violet Safranin O | 71.4 95.3 80.9 86.1 | [133] |
| Fe3O4-Coffe Skin | - | - | Methylene blue | 556 | [134] |
| Fe3O4-Ground coffe waste | - | - | Methylene blue | 128 | [135] |
| Fe3O4-Starch Fe3O4-Av1-Starch Fe3O4-Wm-Starch | 62 78 63 | 51 43 49 | Optilan blue | 119 111 63 | [136] |
| Fe3O4-Red mud | 84 | 12 | Diazinon Malathion Parathiion Chlorpyrifos | 1.9 1.7 2.9 3.9 | [137] |
| Fe3O4-Red mud | 84 | 12 | Ciprofloxacin | 200 | [138] |
| Fe3O4-Tea Waste | - | - | Neutral red Reactive blue Congo red Janus green Methilene blue Crystal violet Thionine | 127 88 83 130 119 114 128 | [139] |
| Fe3O4-Tree leaves | - | - | Malachite green Neutral red Methylene blue Crystal violet Methyl violet | 89 101 128 117 133 | [140] |
| Fe3O4-Olive cristal cellulose | - | 21 | Methylene blue | 166 | [124] |
| Fe3O4-Acuatic Plant | 7 | 3 | Methylene blue | 474 | [141] |
| Fe3O4-Beer Yeast | - | - | Methylene blue Basic violet | 609 521 | [142] |
| Fe3O4-SBO | 35 | 51 | Crystal violet | 244 | [143] |
| Fe3O4 (MP-10) | 70 | 9 | Methylene blue | 99.4 | [144] |
| Fe3O4 (MP-3) Fe3O4 (MP-3w) | 176 119 | 5 4 | Methylene blue | 87.3 56.7 | [146] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peralta, M.E.; Ocampo, S.; Funes, I.G.; Onaga Medina, F.; Parolo, M.E.; Carlos, L. Nanomaterials with Tailored Magnetic Properties as Adsorbents of Organic Pollutants from Wastewaters. Inorganics 2020, 8, 24. https://doi.org/10.3390/inorganics8040024
Peralta ME, Ocampo S, Funes IG, Onaga Medina F, Parolo ME, Carlos L. Nanomaterials with Tailored Magnetic Properties as Adsorbents of Organic Pollutants from Wastewaters. Inorganics. 2020; 8(4):24. https://doi.org/10.3390/inorganics8040024
Chicago/Turabian StylePeralta, Marcos E., Santiago Ocampo, Israel G. Funes, Florencia Onaga Medina, María E. Parolo, and Luciano Carlos. 2020. "Nanomaterials with Tailored Magnetic Properties as Adsorbents of Organic Pollutants from Wastewaters" Inorganics 8, no. 4: 24. https://doi.org/10.3390/inorganics8040024
APA StylePeralta, M. E., Ocampo, S., Funes, I. G., Onaga Medina, F., Parolo, M. E., & Carlos, L. (2020). Nanomaterials with Tailored Magnetic Properties as Adsorbents of Organic Pollutants from Wastewaters. Inorganics, 8(4), 24. https://doi.org/10.3390/inorganics8040024