A Partial Anion Disorder in SrVO2H Induced by Biaxial Tensile Strain
Abstract
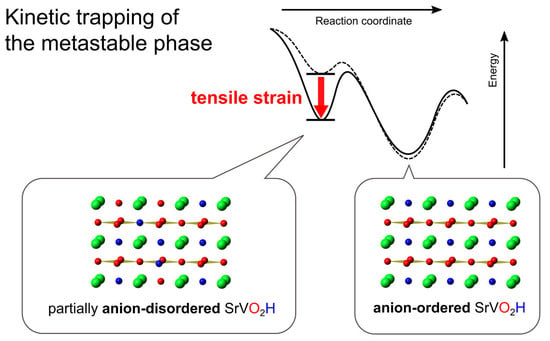
:1. Introduction
2. Results and Discussion
2.1. Low-Temperature Reduction of SrVO3 Films
2.2. Structural CharacterizatIon
2.3. The Role of Tensile Strain
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kageyama, H.; Hayashi, K.; Maeda, K.; Attfield, J.P.; Hiroi, Z.; Rondinelli, J.M.; Poeppelmeier, K.R. Expanding frontiers in materials chemistry and physics with multiple anions. Nat. Commun. 2018, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Oró-Solé, J.; Rodgers, J.A.; Jorge, A.B.; Fuertes, A.; Attfield, J.P. Anion order in perovskite oxynitrides. Nat. Chem. 2011, 3, 47–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, K.; Stoltzfus, M.W.; Kim, Y.I.; Proffen, T.; Woodward, P.M.; Cheetham, A.K.; Seshadri, R. Local atomic ordering in BaTaO2N studied by neutron pair distribution function analysis and density functional theory. Chem. Mater. 2007, 19, 4037–4042. [Google Scholar] [CrossRef]
- Camp, P.J.; Fuertes, A.; Attfield, J.P. Subextensive entropies and open order in perovskite oxynitrides. J. Am. Chem. Soc. 2012, 134, 6762–6766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pauling, L. The structure and entropy of ice and of other crystals with some randomness of atomic arrangement. J. Am. Chem. Soc. 1935, 57, 2680–2684. [Google Scholar] [CrossRef]
- Goto, Y.; Tassel, C.; Noda, Y.; Hernandez, O.; Pickard, C.J.; Green, M.A.; Sakaebe, H.; Taguchi, N.; Uchimoto, Y.; Kobayashi, Y.; et al. Pressure-stabilized cubic perovskite oxyhydride BaScO2H. Inorg. Chem. 2017, 56, 4840–4845. [Google Scholar] [CrossRef]
- Blakely, C.K.; Davis, J.D.; Bruno, S.R.; Kraemer, S.K.; Zhu, M.; Ke, X.; Bi, W.; Alp, E.E.; Poltavets, V.V. Multistep synthesis of the SrFeO2F perovskite oxyfluoride via the SrFeO2 infinite-layer intermediate. J. Fluor. Chem. 2014, 159, 8–14. [Google Scholar] [CrossRef]
- Denis Romero, F.; Leach, A.; Möller, J.S.; Foronda, F.; Blundell, S.J.; Hayward, M.A. Strontium vanadium oxide-hydrides: “square-planar” two-electron phases. Angew. Chem. Int. Ed. 2014, 53, 7556–7559. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Zeng, D.; Kawakami, T.; Arcisauskaite, V.; Yata, K.; Patino, M.A.; Izumo, N.; McGrady, J.E.; Kageyama, H.; Hayward, M.A. The role of π-blocking hydride ligands in a pressure-induced insulator-to-metal phase transition in SrVO2H. Nat. Commun. 2017, 8, 1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Y.; Gui, H.; Li, X.; Zhao, Z.; Zhao, Y.H.; Xie, W. The effect of hydrogen ordering on the electronic and magnetic properties of the strontium vanadium oxyhydride. J. Phys. Condens. Matter 2015, 27, 206001. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Shitara, K.; Kitagawa, S.; Kuwabara, A.; Kuroe, M.; Ishida, K.; Ochi, M.; Kuroki, K.; Fujii, K.; Yashima, M.; et al. Selective hydride occupation in BaVO3−xHx (0.3 ≤ x ≤ 0.8) with face and corner-shared octahedra. Chem. Mater. 2018, 30, 1566–1574. [Google Scholar] [CrossRef]
- Oka, D.; Hirose, Y.; Kamisaka, H.; Fukumura, T.; Sasa, K.; Ishii, S.; Matsuzaki, H.; Sato, Y.; Ikuhara, Y.; Hasegawa, T. Possible ferroelectricity in perovskite oxynitride SrTaO2N epitaxial thin films. Sci. Rep. 2014, 4, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Oka, D.; Hirose, Y.; Matsui, F.; Kamisaka, H.; Oguchi, T.; Maejima, N.; Nishikawa, H.; Muro, T.; Hayashi, K.; Hasegawa, T. Strain engineering for anion arrangement in perovskite oxynitrides. ACS Nano 2017, 11, 3860–3866. [Google Scholar] [CrossRef] [PubMed]
- Katayama, T.; Chikamatsu, A.; Yamada, K.; Shigematsu, K.; Onozuka, T.; Minohara, M.; Kumigashira, H.; Ikenaga, E.; Hasegawa, T. Epitaxial growth and electronic structure of oxyhydride SrVO2H thin films. J. Appl. Phys. 2016, 120, 085305. [Google Scholar] [CrossRef]
- Rey, M.J.; Dehaudt, P.; Joubert, J.C.; Lambert-Andron, B.; Cyrot, M.; Cyrot-Lackmann, F. Preparation and structure of the compounds SrVO3 and Sr2VO4. J. Solid State Chem. 1990, 86, 101–108. [Google Scholar] [CrossRef]
- Folkman, C.M.; Baek, S.H.; Jang, H.W.; Eom, C.B.; Nelson, C.T.; Pan, X.Q.; Li, Y.L.; Chen, L.Q.; Kumar, A.; Gopalan, V.; et al. Stripe domain structure in epitaxial (001) BiFeO3 thin films on orthorhombic TbScO3 substrate. Appl. Phys. Lett. 2009, 94, 251911. [Google Scholar] [CrossRef] [Green Version]
- Katayama, T.; Chikamatsu, A.; Kamisaka, H.; Yokoyama, Y.; Hirata, Y.; Wadati, H.; Fukumura, T.; Hasegawa, T. Topotactic synthesis of strontium cobalt oxyhydride thin film with perovskite structure. AIP Adv. 2015, 5, 107147. [Google Scholar] [CrossRef]
- Kawai, M.; Matsumoto, K.; Ichikawa, N.; Mizumaki, M.; Sakata, O.; Kawamura, N.; Kimura, S.; Shimakawa, Y. Orientation change of an infinite-layer structure LaNiO2 epitaxial thin film by annealing with CaH2. Cryst. Growth Des. 2010, 10, 2044–2046. [Google Scholar] [CrossRef]
- Yajima, T.; Kitada, A.; Kobayashi, Y.; Sakaguchi, T.; Bouilly, G.; Kasahara, S.; Terashima, T.; Takano, M.; Kageyama, H. Epitaxial thin films of ATiO3−xHx (A = Ba, Sr, Ca) with metallic conductivity. J. Am. Chem. Soc. 2012, 134, 8782–8785. [Google Scholar] [CrossRef]
- Bouilly, G.; Yajima, T.; Terashima, T.; Kususe, Y.; Fujita, K.; Tassel, C.; Yamamoto, T.; Tanaka, K.; Kobayashi, Y.; Kageyama, H. Substrate-induced anion rearrangement in epitaxial thin films of LaSrCoO4–xHx. CrystEngComm 2014, 16, 9669–9674. [Google Scholar] [CrossRef]
- Yamamoto, T.; Ohkubo, H.; Tassel, C.; Hayashi, N.; Kawasaki, S.; Okada, T.; Yagi, T.; Hester, J.; Avdeev, M.; Kobayashi, Y.; et al. Impact of lanthanoid substitution on the structural and physical properties of an infinite-layer iron oxide. Inorg. Chem. 2016, 55, 12093–12099. [Google Scholar] [CrossRef]
- Patino, M.A.; Zeng, D.; Blundell, S.J.; McGrady, J.E.; Hayward, M.A. Extreme sensitivity of a topochemical reaction to cation substitution: SrVO2H versus SrV1−xTixO1.5H1.5. Inorg. Chem. 2018, 57, 2890–2898. [Google Scholar] [CrossRef] [PubMed]
- Aschauer, U.; Pfenninger, R.; Selbach, S.M.; Grande, T.; Spaldin, N.A. Strain-controlled oxygen vacancy formation and ordering in CaMnO3. Phys. Rev. B 2013, 88, 054111. [Google Scholar] [CrossRef] [Green Version]
- Kutsuzawa, D.; Hirose, Y.; Chikamatsu, A.; Nakao, S.; Watahiki, Y.; Harayama, I.; Sekiba, D.; Hasegawa, T. Strain-enhanced topotactic hydrogen substitution for oxygen in SrTiO3 epitaxial thin film. Appl. Phys. Lett. 2018, 113, 253104. [Google Scholar] [CrossRef]
- Hayward, M.A.; Rosseinsky, M.J. Materials chemistry: Cool conditions for mobile ions. Nature 2007, 450, 960–961. [Google Scholar] [CrossRef]
- Prado, F.; Mogni, L.; Cuello, G.J.; Caneiro, A. Neutron powder diffraction study at high temperature of the Ruddlesden-Popper phase Sr3Fe2O6+δ. Solid State Ion. 2007, 178, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Kobayashi, Y.; Shitara, K.; Konishi, A.; Kuwabara, A.; Nakashima, T.; Tassel, C.; Yamamoto, T.; Kageyama, H. On hydride diffusion in transition metal perovskite oxyhydrides investigated via deuterium exchange. Chem. Mater. 2017, 29, 8187–8194. [Google Scholar] [CrossRef]
- Bither, T.A.; Prewitt, C.T.; Gillson, J.L.; Bierstedt, P.E.; Flippen, R.B.; Young, H.S. New transition metal dichalcogenides formed at high pressure. Solid State Commun. 1966, 4, 533–535. [Google Scholar] [CrossRef]
- Bither, T.A.; Bouchard, R.J.; Cloud, W.H.; Donohue, P.C.; Siemons, W.J. Transition metal pyrite dichalcogenides. High-pressure synthesis and correlation of properties. Inorg. Chem. 1968, 7, 2208–2220. [Google Scholar] [CrossRef]
- Martinolich, A.J.; Neilson, J.R. Toward Reaction-by-Design: Achieving Kinetic Control of Solid State Chemistry with Metathesis. Chem. Mater. 2017, 29, 479–489. [Google Scholar] [CrossRef]
- Ni, J.; Gu, B. The metastable phase diagram and the kinetics of transient ordered states in a ternary system. J. Phys. Condens. Matter 1998, 10, 3523–3534. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namba, M.; Takatsu, H.; Yoshimune, W.; Daniel, A.; Itoh, S.; Terashima, T.; Kageyama, H. A Partial Anion Disorder in SrVO2H Induced by Biaxial Tensile Strain. Inorganics 2020, 8, 26. https://doi.org/10.3390/inorganics8040026
Namba M, Takatsu H, Yoshimune W, Daniel A, Itoh S, Terashima T, Kageyama H. A Partial Anion Disorder in SrVO2H Induced by Biaxial Tensile Strain. Inorganics. 2020; 8(4):26. https://doi.org/10.3390/inorganics8040026
Chicago/Turabian StyleNamba, Morito, Hiroshi Takatsu, Wataru Yoshimune, Aurélien Daniel, Shoichi Itoh, Takahito Terashima, and Hiroshi Kageyama. 2020. "A Partial Anion Disorder in SrVO2H Induced by Biaxial Tensile Strain" Inorganics 8, no. 4: 26. https://doi.org/10.3390/inorganics8040026
APA StyleNamba, M., Takatsu, H., Yoshimune, W., Daniel, A., Itoh, S., Terashima, T., & Kageyama, H. (2020). A Partial Anion Disorder in SrVO2H Induced by Biaxial Tensile Strain. Inorganics, 8(4), 26. https://doi.org/10.3390/inorganics8040026