Facile Synthesis and Redox Behavior of an Overcrowded Spirogermabifluorene
Abstract
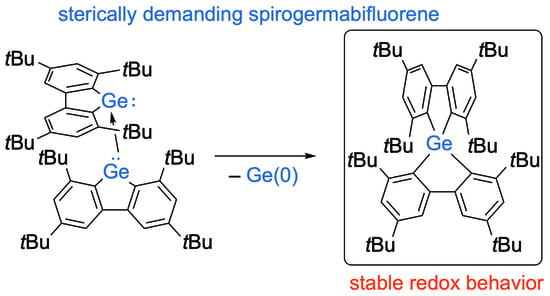
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. General Information
4.2. Synthesis of Dichlorogermane 5
4.3. Synthesis of Spirogermabifluorene 1
4.4. X-ray Crystallographic Analysis of Spirogermabifluorene 1 and Dichlorogermane 5
4.5. Electrochemical Measurements of Spirogermabifluorene 1
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References and Notes
- Suzuki, T.; Takahashi, H.; Nishida, J.-i.; Tsuji, T. Biphenyl-type electron acceptors exhibiting dynamic redox properties: A novel electrochromic system with ‘write protect’ option. Chem. Commun. 1998, 1331–1332. [Google Scholar] [CrossRef]
- Luo, P.; Feinberg, E.C.; Guirado, G.; Farid, S.; Dinnocenzo, J.P. Accurate Oxidation Potentials of 40 Benzene and Biphenyl Derivatives with Heteroatom Substituents. J. Org. Chem. 2014, 79, 9297–9304. [Google Scholar] [CrossRef]
- Pan, F.; Yang, J.; Jia, C.; Li, H.; Wang, Q. Biphenyl-lithium-TEGDME solution as anolyte for high energy density non-aqueous redox flow lithium battery. J. Energy Chem. 2018, 27, 1362–1368. [Google Scholar] [CrossRef] [Green Version]
- Clarkson, R.G.; Gomberg, M. Spirans with four aromatic radicals on the spiro carbon atom. J. Am. Chem. Soc. 1930, 52, 2881–2891. [Google Scholar] [CrossRef]
- Simmons, H.E.; Fukunaga, T. Spiroconjugation. J. Am. Chem. Soc. 1967, 89, 5208–5215. [Google Scholar] [CrossRef]
- Hoffmann, R.; Imamura, A.; Zeiss, G.D. The Spirarenes. J. Am. Chem. Soc. 1967, 89, 5215–5220. [Google Scholar] [CrossRef]
- Semmelhack, M.F.; Foos, J.S.; Katz, S. Spiro[4.4]nonatetraene and Spiro[4.4]nona-l,3,7-triene. Synthesis and Properties. Effects of Spiroconjugation. J. Am. Chem. Soc. 1973, 95, 7325–7336. [Google Scholar] [CrossRef]
- Yin, H.; Xiao, H.; Ding, L.; Zhang, C.; Ren, A.; Li, B. A bistriphenylamine-substituted spirobifluorene derivative exhibiting excellent nonlinearity/transparency/thermal stability trade-off and strong two-photon induced blue fluorescence. Mater. Chem. Phys. 2015, 151, 181–186. [Google Scholar] [CrossRef]
- Zhu, K.; Kamochi, K.; Kodama, T.; Tobisu, M.; Amaya, T. Chiral cyclic [n]spirobifluorenylenes: Carbon nanorings consisting of helically arranged quaterphenyl rods illustrating partial units of woven patterns. Chem. Sci. 2020, 11, 9604–9610. [Google Scholar] [CrossRef]
- Oniki, J.; Moriuchi, T.; Kamochi, K.; Tobisu, M.; Amaya, T. Linear [3] Spirobifluorenylene: An S-Shaped Molecular Geometry of p-Oligophenyls. J. Am. Chem. Soc. 2019, 141, 18238–18245. [Google Scholar] [CrossRef]
- Gilman, H.; Gorsich, R.D. Cyclic Organosilicon Compounds. I. Synthesis of Compounds Containing the Dibenzosilole Nucleus. J. Am. Chem. Soc. 1958, 80, 1883–1886. [Google Scholar] [CrossRef]
- Gelius, R. Dibenzostannole und ihre Abbauprodukte. Chem. Ber. 1960, 93, 1883–1886. [Google Scholar] [CrossRef]
- Cohen, S.C.; Massey, A.C. 3,3’,4,4’,5,5’,6,6’-OCTARLUOROBIPHEBYL. Tetrahedron Lett. 1966, 37, 4393–4394. [Google Scholar] [CrossRef]
- Boo, B.H.; Park, J.; Yeo, H.G.; Lee, S.Y.; Park, C.J.; Kim, J.H. Infrared and Raman Spectroscopy of 9,9’-Spirobifluorene, Bis(2,2’-biphenylene)silane, and Bis(2,2’-biphenylene)germane. Vibrational Assignment by Depolarization Measurement and HF and Density Functional Theory Studies. J. Phys. Chem. A 1998, 102, 1139–1145. [Google Scholar] [CrossRef]
- Saito, M.; Imaizumi, S.; Tajima, T.; Ishimura, K.; Nagase, S. Synthesis and structure of pentaorganostannate having five carbon substituents. J. Am. Chem. Soc. 2007, 129, 10974–10975. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Imaizumi, S.; Tajima, T. Formation of Pentaorganostannates from Bis(2-bromo-2-biphenyl)stannanes and tert-Butyllithium upon Substitution of Alkyl and Aryl Groups on Tin Atoms. Eur. J. Inorg. Chem. 2010, 2010, 2153–5157. [Google Scholar] [CrossRef]
- van der Boon, L.J.P.; van Gelderen, L.; de Groot, T.R.; Lutz, M.; Slootweg, J.C.; Ehlers, A.W.; Lammertsma, K. Chiral Control in Pentacoordinate Systems: The Case of Organosilicates. Inorg. Chem. 2018, 57, 12697–12708. [Google Scholar] [CrossRef] [PubMed]
- Aspin, S.J.; Taillemaud, S.; Cyr, P.; Charette, A.B. 9-Silafluorenyl Dichlorides as Chemically Ligating Coupling Agents and Their Application in Peptide Synthesis. Angew. Chem. Int. Ed. 2016, 55, 13833–13837. [Google Scholar] [CrossRef]
- Couzijn, E.P.; van den Engel, D.W.; Slootweg, J.C.; de Kanter, F.J.; Ehlers, A.W.; Schakel, M.; Lammertsma, K. Configurationally rigid pentaorganosilicates. J. Am. Chem. Soc. 2009, 131, 3741–3751. [Google Scholar] [CrossRef]
- Zabula, A.V.; Dolinar, B.S.; West, R. Transformations of spirogermabifluorene upon reduction with alkali metals. J. Organomet. Chem. 2014, 751, 458–461. [Google Scholar] [CrossRef]
- Fischer, R.C.; Power, P.P. π-Bonding and the Lone Pair Effect in Multiple Bonds Involving Heavier Main Group Elements: Developments in the New Millennium. Chem. Rev. 2010, 110, 3877–3923. [Google Scholar] [CrossRef]
- Suzuki, Y.; Sasamori, T.; Guo, J.-D.; Tokitoh, N. A Redox-Active Bis(ferrocenyl)germylene and Its Reactivity. Chem.–Eur. J. 2018, 24, 364–368. [Google Scholar] [CrossRef]
- Sakagami, M.; Sasamori, T.; Sakai, H.; Furukawa, Y.; Tokitoh, N. 1,2-Bis(ferrocenyl)dipnictenes: Bimetallic Systems with a Pn=Pn Heavy π-Spacer (Pn: P, Sb, and Bi). Bull. Chem. Soc. Jpn. 2013, 86, 1132–1143. [Google Scholar] [CrossRef] [Green Version]
- Sasamori, T.; Mieda, E.; Nagahora, N.; Sato, K.; Shiomi, D.; Takui, T.; Hosoi, Y.; Furukawa, Y.; Takagi, N.; Nagase, S.; et al. One-Electron Reduction of Kinetically Stabilized Dipnictenes: Synthesis of Dipnictene Anion Radicals. J. Am. Chem. Soc. 2006, 128, 12582–12588. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.G.; Spencer, N.S.; Philp, D.; Kariuki, B.M.; Snaith, J.S. The First Spirobifluorenes Containing Two Binaphthyl Moieties. Organometallics 2003, 22, 5589–5592. [Google Scholar] [CrossRef]
- Winkel, Y.V.D.; Laarse, J.V.D.; Kanter, F.J.J.D.; Does, T.V.D.; Bickelhaupt, F.; Smeets, W.J.J.; Spek, A.L. Investigations of a highly crowded phosphino-substituted biphenyl: A precursor for a λ3, λ5-diphosphaphenanthrene. Heteroat. Chem. 1991, 2, 17–28. [Google Scholar] [CrossRef]
- Data for 2,2′-dimethyl-3,3′5,5′-tetra-tert-butylbiphenyl, which is formed by the reaction of 4 with MeI: Colorless solid, mp. 164-166 °C. 1H NMR (500 MHz, CDCl3) δ1.41 (s, 18H), 1.49 (s 18H), 2.11 (s 6H), 7.05 (d 2H), 7.46 (d 2H); 13C NMR (126 MHz, CDCl3) δ148.0, 145.9, 131,7, 125.1, 122.4, 36.9, 35. 3, 32.2, 32.0, 19.8.
- The differential UV/vis spectrum is shown in the Supporting Information. The strong absorptions for 6A and 6B were estimated as λmax = 397 nm (f = 0.1219; 6A) and λmax = 423 nm (f = 0.1243; 6B) based on TDDFT calculations at the TD-B3PW91/6-311G(3df)[Ge], 6-311G(d)[C,H]//B3PW91/6-311G(3d)[Ge], 6-31G(d)[C,H] level of theory.
- Dibromogermane 7 could not be separated and purified from the by-products including 2 and other unidentified compounds by GPC and/or column chromatography due to its low stability. It was characterized only by 1H NMR and HRMS of the crude reaction mixture. 7: 1H NMR (500 MHz, C6D6) δ 7.95 (d, 4J = 2.3 Hz, 2H), 7.68 (d, 4J = 2.3 Hz, 2H), 1.67 (s, 18H), 1.36 (s, 18H); HRMS (DART-TOF), m/z: Found: 611.0758 ([M+H]+), Calcd. for C28H4181Br272Ge ([M+H]+): 611.0771.
- The molecular geometries for the transition states were first estimated using the Reaction plus software package (software to optimize reaction paths along the user’s expected ones, HPC Systems Inc. Available online: http://www.hpc.co.jp/chem/react2.html (accessed on 16 September 2021). written in Japanese), based on the nudged-elastic-band (NEB) method (Henkelman, G.; Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 2000, 113, 9978–9985. [Google Scholar] [CrossRef] and subsequently re-optimized using the Gaussian 16 software package.
- Baines, K.M.; Cooke, J.A.; Vittal, J.J. A Facile Digermene-to-germylgermylene Rearrangement; Bulky Germylene Insertion into the Si-H Bond. J. Chem. Soc. Chem. Commun. 1992, 1484–1485. [Google Scholar] [CrossRef]
- Crystallographic data for 1 (CCDC-2106926): C56H80Ge, FW 825.79, crystal size 0.10×0.10×0.08 mm3, temperature −180 °C, tetragonal, space group P41 (#76), a = 11.0197(1) Å, b = 11.0197(1) Å, c = 39.7580(7) Å, α = β = γ = 90°, V = 4827.96(12) Å3, Z = 4, Dcalcd = 1.136 g cm−3, μ = 0.669 mm−1, θmax = 26.483°, 92883 reflections measured, 9991 independent reflections (Rint = 0.1245), 563 parameters refined, GOF = 1.123, completeness = 99.8%, R1 [I > 2σ(I)] = 0.0542, wR2 (all data) = 0.1276, largest diff. peak and hole 1.595 and −0.492 e Å−3. 5 (CCDC-2106927): C28H40Cl2Ge, FW 520.09, crystal size 0.20 × 0.10 × 0.01 mm3, temperature −170 °C, orthorhombic, space group P212121 (#19), a = 12.3031(2) Å, b = 13.7729(2) Å, c = 16.2300(3) Å, α = β = γ = 90°, V = 2750.16(8) Å3, Z = 4, Dcalcd = 1.256 g cm−3, μ = 1.321 mm−1, θmax = 26.495°, 54467 reflections measured, 5655 independent reflections (Rint = 0.0456), 323 parameters refined, GOF = 1.137, completeness = 99.1%, R1 [I > 2σ(I)] = 0.0291, wR2 (all data) = 0.0696, largest diff. peak and hole 0.358 and −0.305 e Å−3.
- Zabula, A.V.; Rogachev, A.Y.; Guzei, I.A.; West, R. Silicon in a Negatively Charged Shell: Anions of Spirosilabifluorene. Organometallics 2013, 32, 3760–3768. [Google Scholar] [CrossRef]
- The theoretically optimized structure of the dianionic species of 1 that presumes an intact spirogermabifluorene skeleton is shown in the Supporting Information.







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morisako, S.; Noro, K.; Sasamori, T. Facile Synthesis and Redox Behavior of an Overcrowded Spirogermabifluorene. Inorganics 2021, 9, 75. https://doi.org/10.3390/inorganics9100075
Morisako S, Noro K, Sasamori T. Facile Synthesis and Redox Behavior of an Overcrowded Spirogermabifluorene. Inorganics. 2021; 9(10):75. https://doi.org/10.3390/inorganics9100075
Chicago/Turabian StyleMorisako, Shogo, Kohsuke Noro, and Takahiro Sasamori. 2021. "Facile Synthesis and Redox Behavior of an Overcrowded Spirogermabifluorene" Inorganics 9, no. 10: 75. https://doi.org/10.3390/inorganics9100075
APA StyleMorisako, S., Noro, K., & Sasamori, T. (2021). Facile Synthesis and Redox Behavior of an Overcrowded Spirogermabifluorene. Inorganics, 9(10), 75. https://doi.org/10.3390/inorganics9100075