Effects of Protective Surface Coating on Fluoride Release and Recharge of Recent Uncoated High-Viscosity Glass Ionomer Cement
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
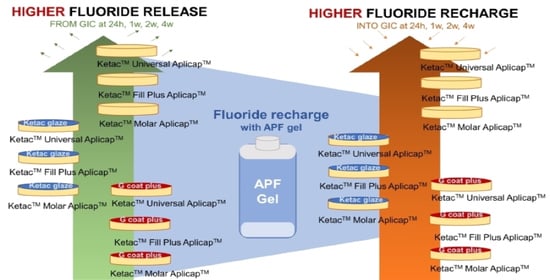
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wilson, A.D.; Kent, B.E. A new translucent cement for dentistry. The glass ionomer cement. Br. Dent. J. 1972, 132, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Menezes-Silva, R.; Cabral, R.N.; Pascotto, R.C.; Borges, A.F.S.; Martins, C.C.; Navarro, M.F.L.; Sidhu, S.K.; Leal, S.C. Mechanical and optical properties of conventional restorative glass-ionomer cements—A systematic review. J. Appl. Oral. Sci. 2019, 27, e2018357. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.K.; Nicholson, J.W. A Review of Glass-Ionomer Cements for Clinical Dentistry. J. Funct. Biomater. 2016, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Bonifácio, C.C.; Werner, A.; Kleverlaan, C.J. Coating glass-ionomer cements with a nanofilled resin. Acta Odontol. Scand. 2012, 70, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, E.; Bossù, M.; Giovannetti, A.; La Torre, G.; Guerra, F.; Polimeni, A. Surface roughness of glass ionomer cements indicated for uncooperative patients according to surface protection treatment. Ann. Stomatol. 2013, 4, 250–258. [Google Scholar]
- Somani, R.; Jaidka, S.; Singh, D.J.; Sibal, G.K. Comparative Evaluation of Shear Bond Strength of Various Glass Ionomer Cements to Dentin of Primary Teeth: An in vitro Study. Int. J. Clin. Pediatr. Dent. 2016, 9, 192–196. [Google Scholar] [CrossRef]
- Garain, R.; Abidi, M.; Mehkri, Z. Compressive and Flexural Strengths of High-strength Glass Ionomer Cements: A Systematic Review. Int. J. Exp. Dent. Sci. 2020, 9, 25–29. [Google Scholar] [CrossRef]
- Hesse, D.; Bonifácio, C.C.; Kleverlaan, C.J.; Raggio, D.P. Clinical wear of approximal glass ionomer restorations protected with a nanofilled self-adhesive light-cured protective coating. J. Appl. Oral. Sci. 2018, 26, e20180094. [Google Scholar] [CrossRef] [Green Version]
- Thongbai-On, N.; Banomyong, D. Flexural strengths and porosities of coated or uncoated, high powder-liquid and resin-modified glass ionomer cements. J. Dent. Sci. 2020, 15, 433–436. [Google Scholar] [CrossRef]
- Brzović Rajić, V.; Ivanišević Malčić, A.; Bilge Kütük, Z.; Gurgan, S.; Jukić, S.; Miletić, I. Compressive Strength of New Glass Ionomer Cement Technology based Restorative Materials after Thermocycling and Cyclic Loading. Acta Stomatol. Croat. 2019, 53, 318–325. [Google Scholar] [CrossRef]
- Ugurlu, M. Effects of surface coating on the flexural strength of fluoridereleasing restorative materials after water aging for one year. Eur. Oral. Res. 2020, 54, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Kishore, G.; Sai-Sankar, A.J.; Pratap-Gowd, M.; Sridhar, M.; Pranitha, K.; Sai-Krishna, V.S. Comparative Evaluation of Fluoride Releasing Ability of Various Restorative Materials after the Application of Surface Coating Agents—An In-vitro Study. J. Clin. Diagn. Res. 2016, 10, ZC38–ZC41. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.I.; Yassen, A.A.; Bayoumi, R.E. Influence of Nanocoats on the Physicomechanical Properties and Microleakage of Bulk-fill and Resin-modified Glass Ionomer Cements: An In Vitro Study. J. Contemp. Dent. Pract. 2021, 22, 62–68. [Google Scholar] [CrossRef]
- Francois, P.; Fouquet, V.; Attal, J.-P.; Dursun, E. Commercially Available Fluoride-Releasing Restorative Materials: A Review and a Proposal for Classification. Materials 2020, 13, 2313. [Google Scholar] [CrossRef] [PubMed]
- Frankenberger, R.; Sindel, J.; Krämer, N. Viscous glass-ionomer cements: A new alternative to amalgam in the primary dentition? Quintessence Int. 1997, 28, 667–676. [Google Scholar] [PubMed]
- Park, E.Y.; Kang, S. Current aspects and prospects of glass ionomer cements for clinical dentistry. Yeungnam Univ. J. Med. 2020, 37, 169–178. [Google Scholar] [CrossRef]
- Aydın, N.; Karaoğlanoğlu, S.; Aybala-Oktay, E.; Çetinkaya, S.; Erdem, O. Investigation of water sorption and aluminum releases from high viscosity and resin modified glass ionomer. J. Clin. Exp. Dent. 2020, 12, e844–e851. [Google Scholar] [CrossRef]
- Ryu, W.; Park, H.; Lee, J.; Seo, H. Effect of Nano-filled Protective Coating on Microhardness and Wear Resistance of Glass-ionomer Cements. J. Korean Acad. Pediatr. Dent. 2019, 46, 226–232. [Google Scholar] [CrossRef]
- Shintome, L.K.; Nagayassu, M.P.; Di Nicoló, R.; Myaki, S.I. Microhardness of glass ionomer cements indicated for the ART technique according to surface protection treatment and storage time. Braz. Oral. Res. 2009, 23, 439–445. [Google Scholar] [CrossRef] [Green Version]
- Fuhrmann, D.; Murchison, D.; Whipple, S.; Vandewalle, K. Properties of new glass-ionomer restorative systems marketed for stress-bearing areas. Oper. Dent. 2020, 45, 104–110. [Google Scholar] [CrossRef]
- Sukumaran, V.G.; Rathakrishnan, M. To Evaluate the Effect of Surface Coating on Three Different Types Glass Ionomer Restorations. Biomed. Pharmacol. J. 2015, 8, 445–449. [Google Scholar] [CrossRef]
- Fatima, N.; Ali Abidi, S.Y.; Qazi, F.U.; Jat, S.A. Effectiveness of commonly available surface protecting agents in maintaining microhardness of two cements. J. Coll. Physicians Surg. Pak. 2013, 23, 315–318. [Google Scholar]
- Kelić, K.; Par, M.; Peroš, K.; Šutej, I.; Tarle, Z. Fluoride-releasing restorative materials: The effect of a resinous coat on ion release. Acta Stomatol. Croat. 2020, 54, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Habib, S. Fluoride releasing/recharging ability of bulk-fill and resin modified glass ionomer cements after the application of different surface coating agents: An in -vitro study. Adv. Dent. J. 2020, 2, 80–92. [Google Scholar] [CrossRef]
- Brzović-Rajić, V.; Miletić, I.; Gurgan, S.; Peroš, K.; Verzak, Ž.; Ivanišević-Malčić, A. Fluoride release from glass ionomer with nano filled coat and varnish. Acta Stomatol. Croat. 2018, 52, 307–313. [Google Scholar] [CrossRef]
- Tiwari, S.; Nandlal, B. Effect of nano-filled surface coating agent on fluoride release from conventional glass ionomer cement: An in vitro trial. J. Indian Soc. Pedod. Prev. Dent. 2013, 31, 91–95. [Google Scholar] [CrossRef]
- Hattab, F.N.; Amin, W.M. Fluoride release from glass ionomer restorative materials and the effects of surface coating. Biomaterials 2001, 22, 1449–1458. [Google Scholar] [CrossRef]
- Ahn, C.; Bair, J.; Lee, E.D.; Finkelman, M.; Harsono, M.; Perry, R.; Kugel, G. Fluoride Release from Uncoated and Coated Glass Ionomer Materials. In Proceedings of the 2013 IADR/AADR/CADR General Session, Seattle, WA, USA, 20–23 March 2013. [Google Scholar]
- Auychai, P.; Khumtrakoon, N.; Jitongart, C.; Daomanee, P.; Laiteerapong, A. Bond strength and microleakage of a novel glass ionomer cement containing silver diamine fluoride. Eur. J. Dent. 2021, 16, 606–611. [Google Scholar] [CrossRef]
- Soliman, T.; Othman, M. Mechanical properties of the new Ketac™ Universal glass ionomer restorative material: Effect of resin coating. E.D.J. 2017, 63, 1027–1035. [Google Scholar] [CrossRef] [Green Version]
- Mohamed Abdel-Hamid, D.; Mohamed, G.; El-sharkawy, F.; Abou-Auf, E. Effect of surface protection, staining beverages and aging on the color stability and hardness of recently introduced uncoated glass ionomer restorative material. Futur. Dent. J. 2018, 4, 288–296. [Google Scholar] [CrossRef]
- Dulsamphan, C.; Chotitanmapong, T.; Klaisiri, A.; Krajangta, N. Effect of Protective Surface Coating on Hardness of Recent Uncoated High Viscosity Glass Ionomer Cement. J. Int. Dent. 2022, 15, 1036–1042. [Google Scholar]
- ISO19448; Analysis of Fluoride Concentration in Aqueous Solutions by Use of Fluoride Ion-Selective Electrode. International Organization for Standardization: Geneva, Switzerland, 2018.
- Cabral, M.F.C.; de Menezes Martinho, R.L.; Guedes-Neto, M.V.; Rebelo, M.A.B.; Pontes, D.G.; Cohen-Carneiro, F. Do conventional glass ionomer cements release more fluoride than resin-modified glass ionomer cements? Restor. Dent. Endod. 2015, 40, 209–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jingarwar, M.M.; Pathak, A.; Bajwa, N.K.; Sidhu, H.S. Quantitative assessment of fluoride release and recharge ability of different restorative materials in different media: An in vitro study. J. Clin. Diagn. Res. 2014, 8, ZC31–ZC34. [Google Scholar] [CrossRef] [PubMed]
- Dionysopoulos, D. The effect of fluoride-releasing restorative materials on inhibition of secondary caries formation. Fluoride 2014, 47, 258–265. [Google Scholar]
- Kumari, P.D.; Khijmatgar, S.; Chowdhury, A.; Lynch, E.; Chowdhury, C.R. Factors influencing fluoride release in atraumatic restorative treatment (ART) materials: A review. J. Oral. Biol. Craniofac. Res. 2019, 9, 315–320. [Google Scholar] [CrossRef]
- Xu, X.; Burgess, J.O. Compressive strength, fluoride release and recharge of fluoride-releasing materials. Biomaterials 2003, 24, 2451–2461. [Google Scholar] [CrossRef]
- Shatat, F. The Effect of Resin Based Coatings on Fluoride Release of Glass Ionomer Cement, an In Vitro Study; University of the Western Cape: Cape Town, South Africa, 2018. [Google Scholar]
- Nicholson, J.W. Fluoride-releasing dental restorative materials: An update. Balk. J. Dent. Med. 2015, 18, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Faraji, F.; Heshmat, H.; Banava, S. Effect of protective coating on microhardness of a new glass ionomer cement: Nanofilled coating versus unfilled resin. J. Conserv. Dent. 2017, 20, 260–263. [Google Scholar] [CrossRef]
- Rao, B.S.; Moosani, G.K.; Shanmugaraj, M.; Kannapan, B.; Shankar, B.S.; Ismail, P.M. Fluoride release and uptake of five dental restoratives from mouthwashes and dentifrices. J. Int. Oral. Health 2015, 7, 1–5. [Google Scholar]
- Gururaj, M.; Shetty, R.; Nayak, M.; Shetty, S.; Kumar, C.V. Fluoride releasing and Uptake Capacities of Esthetic Restorations. J. Contemp. Dent. Pract. 2013, 14, 887–891. [Google Scholar]
- Dionysopoulos, D.; Koliniotou-Koumpia, E.; Helvatzoglou-Antoniades, M.; Kotsanos, N. Fluoride release and recharge abilities of contemporary fluoride-containing restorative materials and dental adhesives. Dent. Mater. J. 2013, 32, 296–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karabulut, D.; Solak, H. Comparison of fluoride recharge profiles of various restorative materials by linear regression modeling 2011.
- A, G.N.; Jaiswal, J.; Murthy, R.; Pandey, R. Estimation of fluoride release from various dental materials in different media—An in vitro study. Int. J. Clin. Pediatr. Dent. 2009, 2, 1–8. [Google Scholar] [CrossRef]
- Can-Karabulut, D.C.; Batmaz, I.; Solak, H.; Taştekin, M. Linear regression modeling to compare fluoride release profiles of various restorative materials. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2007, 23, 1057–1065. [Google Scholar] [CrossRef] [PubMed]




| Material | Composition |
|---|---|
| KetacTM Fil Plus AplicapTM (CGICs) | Powder: glass powder (>99 wt%) Liquid: water (40–55 wt%), copolymer of acrylic acid–maleic acid (35–55 wt%), tartaric acid (5–10 wt%) Powder/liquid ratio: 3.0/1.0 wt |
| KetacTM Molar AplicapTM | Powder: glass powder (93–98 wt%) Liquid: water (60–65 wt%), copolymer of acrylic acid–maleic acid (30–40 wt%), tartaric acid (5–10 wt%) Powder/liquid ratio: 3.4/1.0 wt |
| KetacTM Universal AplicapTM (HVGICs) | Powder: oxide glass (>95 wt%) Liquid: water (40–60 wt%), copolymer of acrylic acid–maleic acid (30–50 wt%), tartaric acid (1–10 wt%), benzoic acid (<0.2 wt%) Powder/liquid ratio: 3.2/1.0 wt |
| KetacTM Glaze (Unfilled resin coating agent) | Dicyclopentyldimethylene diacrylate (>95 wt%), [(3-methoxypropyl)imino]di-2,1-ethanediyl bismethacrylate (1–5 wt%), 2-[(2-hydroxyethyl)(3-methoxypropyl)amino]ethyl methacrylate (<1 wt%), 2,2- dimethoxy-1,2-diphenylethan-1-one (<0.5 wt%) |
| Materials | Coating | Fluoride Release | Fluoride Recharge | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 1 Week | 2 Weeks | 3 Weeks | 4 Weeks | 24 h | 1 Week | 2 Weeks | 3 Weeks | 4 Weeks | ||
| KetecTM Universal AplicapTM | Uncoated | 27.73 ± 9.35 Aa | 28.27 ± 9.35 Aa | 15.69 ± 6.05 Ab | 9.79 ± 3.92 Ac | 9.35 ± 4.47 Ac | 14.43 ± 6.89 ABb | 14.13 ± 8.88 Abc | 8.18 ± 4.36 Ad | 4.91 ± 2.17 Ae | 4.33 ± 1.72 Af |
| KetacTM Glaze | 5.63 ± 1.17 Ba | 5.92 ± 1.48 BFa | 2.58 ± 0.57 BFb | 2.47 ± 0.6 Bb | 1.8 ± 0.36 Bc | 8.19 ± 0.77 ACd | 2.67 ± 0.29 Bb | 1.37 ± 0.23 BCe | 1.29 ± 0.2 BCf | 0.51 ± 0.1 BCg | |
| G-Coat Plus | 3.49 ± 1.12 Ca | 4.29 ± 1.32 BGa | 2.47 ± 0.75 BFb | 1.52 ± 0.45 CDEc | 1.02 ± 0.3 Cd | 7.18 ± 2.13 Ce | 2.57 ± 0.61 BGHb | 1.28 ± 0.35 BDf | 1.22 ± 0.33 BCf | 0.5 ± 0.15 BCg | |
| KetecTM Molar AplicapTM | Uncoated | 8.95 ± 5.13 BEa | 7.79 ± 4.01 BFEGa | 4.39 ± 2.34 BEFb | 2.61 ± 1.35 BEFGc | 2.66 ± 1.37 BDc | 10.88 ± 3.66 ABCd | 5.15 ± 2.71 ABEFb | 2.18 ± 0.79 CEc | 1.43 ± 0.51 BCe | 1.31 ± 0.45 Df |
| KetacTM Glaze | 2.48 ± 0.64 Ca | 2.53 ± 0.35 Ca | 1.25 ± 0.15 Cb | 1.12 ± 0.12 Cc | 0.83 ± 0.95 Cd | 8.2 ± 1.67 ABCe | 1.91 ± 0.21 Cf | 0.78 ± 0.06 Fd | 0.8 ± 0.09 Dd | 0.35 ± 0.03 Eg | |
| G-Coat Plus | 1.08 ± 0.38 Dae | 1.65 ± 0.36 Db | 0.89 ± 0.19 Da | 0.61 ± 0.13 Hc | 0.5 ± 0.16 Ec | 3.69 ± 0.71 Dd | 1.19 ± 0.17 De | 0.59 ± 0.1 Gc | 0.61 ± 0.09 Ec | 0.21 ± 0.03 Ff | |
| KetecTM Fill Plus AplicapTM | Uncoated | 11.33 ± 3.26 Ea | 12.43 ± 3.56 Eb | 5.76 ± 1.94 Ec | 3.67 ± 0.96 Fd | 3.33 ± 0.9 Dd | 9.27 ± 2.3 ABCe | 4.95 ± 1.56 Ef | 3.05 ± 1.08 Ed | 2.19 ± 0.52 Fg | 1.91 ± 0.88 Dg |
| KetacTM Glaze | 6.09 ± 0.83 Ba | 6.45 ± 0.67 Fa | 2.85 ± 0.22 Bb | 2.33 ± 0.15 Bc | 1.9 ± 0.13 Bd | 9.79 ± 0.99 Be | 3.15 ± 0.27 FGf | 1.49 ± 0.12 Bcg | 1.46 ± 0.12 Bg | 0.57 ± 0.06 Bh | |
| G-Coat Plus | 3.06 ± 1.06 Ca | 4.24 ± 0.98 BGb | 2.21 ± 0.44 Fa | 1.53 ± 0.27 DGc | 1.02 ± 0.19 Cd | 4.21 ± 0.88 Db | 2.11 ± 0.32 CHa | 1.08 ± 0.17 Dd | 1.18 ± 0.14 Cd | 0.43 ± 0.07 Ce | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krajangta, N.; Dulsamphan, C.; Chotitanmapong, T. Effects of Protective Surface Coating on Fluoride Release and Recharge of Recent Uncoated High-Viscosity Glass Ionomer Cement. Dent. J. 2022, 10, 233. https://doi.org/10.3390/dj10120233
Krajangta N, Dulsamphan C, Chotitanmapong T. Effects of Protective Surface Coating on Fluoride Release and Recharge of Recent Uncoated High-Viscosity Glass Ionomer Cement. Dentistry Journal. 2022; 10(12):233. https://doi.org/10.3390/dj10120233
Chicago/Turabian StyleKrajangta, Nantawan, Chayanee Dulsamphan, and Tongjai Chotitanmapong. 2022. "Effects of Protective Surface Coating on Fluoride Release and Recharge of Recent Uncoated High-Viscosity Glass Ionomer Cement" Dentistry Journal 10, no. 12: 233. https://doi.org/10.3390/dj10120233
APA StyleKrajangta, N., Dulsamphan, C., & Chotitanmapong, T. (2022). Effects of Protective Surface Coating on Fluoride Release and Recharge of Recent Uncoated High-Viscosity Glass Ionomer Cement. Dentistry Journal, 10(12), 233. https://doi.org/10.3390/dj10120233