The Stockholm Study: Over 30 years’ Observation of the Effect of Oral Infections on Systemic Health
Abstract
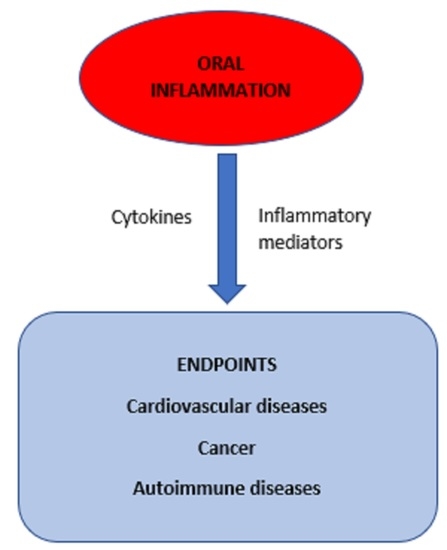
:1. Introduction
2. Studies on the Effect of Tobacco Use
3. Studies on the Effect of Periodontitis, Inflammatory Markers and Cardiovascular Diseases
4. Studies on the Role of Oral Microorganisms and Poor Oral Health
5. Studies on Oral Infections and Cancer
6. Oral Infections and Autoimmune Diseases
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Söder, P.O.; Jin, L.J.; Söder, B.; Wikner, S. Periodontal status in an urban adult population in Sweden. Community Dent. Oral. Epidemiol. 1994, 22, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Söder, P.O.; Söder, B.; Nowak, J.; Jogestrand, T. Early carotid atherosclerosis in subjects with periodontal diseases. Stroke 2005, 36, 1195–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Söder, B.; Yakob, M.; Meurman, J.H.; Andersson, L.C.; Söder, P.Ö. The association of dental plaque with cancer mortality in Sweden. A longitudinal study. BMJ Open 2012, 2, e001083. [Google Scholar] [CrossRef] [PubMed]
- Wickholm, S.; Söder, P.O.; Galanti, M.R.; Söder, B.; Klinge, B. Periodontal disease in a group of Swedish adult snuff and cigarette users. Acta Odontol. Scand. 2004, 62, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Airila-Månsson, S.; Söder, B.; Jin, L.J.; Söder, P.Ö.; Klinge, B. Self-reporting of periodontal diseases and clinical assessment outcome in a Swedish urban population of smokers and non-smokers. Acta Odontol. Scand. 2004, 62, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Julkunen-Iivari, A.; Heikkinen, A.M.; Räisänen, I.T.; Ruokonen, H.; Meurman, J.H.; Toppila-Salmi, S.; Söder, P.Ö.; Söder, B. Tobacco products, periodontal health and education level: Cohort study from Sweden. Dent. J. 2020, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Söder, B.; Airila Månsson, S.; Söder, P.O.; Kari, K.; Meurman, J. Levels of matrix metalloproteinases-8 and -9 with simultaneous presence of periodontal pathogens in gingival crevicular fluid as well as matrix metalloproteinase-9 and cholesterol in blood. J. Periodontal. Res. 2006, 41, 411–417. [Google Scholar] [CrossRef]
- Song, F.; Wisithphrom, K.; Zhou, J.; Windsor, L.J. Matrix metalloproteinase dependent and independent collagen degradation. Front. Biosci. 2006, 11, 3100–3120. [Google Scholar] [CrossRef] [Green Version]
- Schenkein, H.A.; Papapanou, P.N.; Genco, R.; Sanz, M. Mechanisms underlying the association between periodontitis and atherosclerotic disease. Periodontology 2020, 83, 90–106. [Google Scholar] [CrossRef]
- Virtanen, E.; Yakob, M.; Tervahartiala, T.; Söder, P.Ö.; Andersson, L.C.; Sorsa, T.; Meurman, J.H.; Söder, B. Salivary MMP-13 gender differences in periodontitis: A cross-sectional study from Sweden. Clin. Exp. Dent. Res. 2017, 3, 165–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.K.; Suresh, M.V.; Voleti, B.; Agrawal, A. The connection between C-reactive protein and atherosclerosis. Ann. Med. 2008, 40, 110–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakob, M.; Kari, K.; Tervahartiala, T.; Sorsa, T.; Söder, P.Ö.; Meurman, J.H.; Söder, B. Associations of periodontal microorganisms with salivary proteins and MMP-8 in gingival crevicular fluid. J. Clin. Periodontol. 2012, 39, 256–263. [Google Scholar] [CrossRef] [Green Version]
- Yakob, M.; Meurman, J.H.; Jogestrand, T.; Nowak, J.; Söder, P.Ö.; Söder, B. C-reactive protein in relation to early atherosclerosis and periodontitis. Clin. Oral. Investig. 2012, 16, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Söder, P.O.; Meurman, J.H.; Jogestrand, T.; Nowak, J.; Söder, B. Matrix metalloproteinase-9 and tissue inhibitor of matrix metalloproteinase-1 in blood as markers for early atherosclerosis in subjects with chronic periodontitis. J. Periodontal. Res. 2009, 44, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Bäck, M.; Airila-Månsson, S.; Jogestrand, T.; Söder, B.; Söder, P.O. Increased leukotriene concentrations in gingival crevicular fluid from subjects with periodontal disease and atherosclerosis. Atherosclerosis 2007, 193, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Riccioni, G.; Bäck, M.; Capra, V. Leukotrienes and atherosclerosis. Curr. Drug Targets 2010, 11, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Scarmozzino, F.; Poli, A.; Visioli, F. Microbiota and cardiovascular disease risk: A scoping review. Pharm. Res. 2020, 159, 104952. [Google Scholar] [CrossRef]
- Yakob, M.; Söder, B.; Meurman, J.H.; Jogestrand, T.; Nowak, J.; Söder, P.Ö. Prevotella nigrescens and Porphyromonas gingivalis are associated with signs of carotid atherosclerosis in subjects with and without periodontitis. J. Periodontal. Res. 2011, 46, 749–755. [Google Scholar] [CrossRef]
- Meurman, J.H.; Rantonen, P.; Pajukoski, H.; Sulkava, R. Salivary albumin and other constituents and their relation to oral and general health in the elderly. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2002, 94, 432–438. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Yan, H.; Huang, L. Salivary matrix metalloproteinase (MMP)-8 as a biomarker for periodontitis: A PRISMA-compliant systematic review and meta-analysis. Medicine 2018, 97, e9642. [Google Scholar] [CrossRef]
- Söder, B.; Meurman, J.H.; Söder, P.Ö. Gingival inflammation associates with stroke--A role for oral health personnel in prevention: A database study. PLoS ONE 2015, 10, e0137142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Söder, B.; Meurman, J.H.; Söder, P.Ö. Dental calculus links statistically to angina pectoris: 26-year observational study. PLoS ONE 2016, 11, e0157797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Söder, B.; Meurman, J.H.; Söder, P.Ö. Dental calculus is associated with death from heart infarction. Biomed. Res. Int. 2014, 2014, 569675. [Google Scholar] [CrossRef] [PubMed]
- Söder, B.; Jin, L.J.; Klinge, B.; Söder, P.O. Periodontitis and premature death: A 16-year longitudinal study in a Swedish urban population. J. Periodontal. Res. 2007, 42, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, E.; Nurmi, T.; Söder, P.Ö.; Airila-Månsson, S.; Söder, B.; Meurman, J.H. Apical periodontitis associates with cardiovascular diseases: A cross-sectional study from Sweden. BMC Oral. Health 2017, 17, 107. [Google Scholar] [CrossRef] [PubMed]
- Herrera, V.; Parsonnet, J. Helicobacter pylori and gastric adenocarcinoma. Clin. Microbiol. Infect. 2009, 15, 971–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allavena, P.; Garlanda, C.; Borrello, M.G.; Sica, A.; Mantovani, A. Pathways connecting inflammation and cancer. Curr. Opin. Genet. Dev. 2008, 18, 3–10. [Google Scholar] [CrossRef]
- Meurman, J.H. Oral microbiota and cancer. J. Oral. Microbiol. 2010, 2, 5195. [Google Scholar] [CrossRef] [Green Version]
- Söder, B.; Yakob, M.; Meurman, J.H.; Andersson, L.C.; Klinge, B.; Söder, P.Ö. Periodontal disease may associate with breast cancer. Breast. Cancer Res. Treat. 2011, 127, 497–502. [Google Scholar] [CrossRef]
- Söder, B.; Andersson, L.C.; Meurman, J.H.; Söder, P.Ö. Unique database study linking gingival inflammation and smoking in carcinogenesis. Philos. Trans. R. Soc. Lond B Biol. Sci. 2015, 370, 20140041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virtanen, E.; Söder, B.; Andersson, L.C.; Meurman, J.H.; Söder, P.Ö. History of dental infections associates with cancer in periodontally healthy subjects: A 24-year follow-up study from Sweden. J. Cancer 2014, 5, 79–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Söder, B.; Källmén, H.; Yucel-Lindberg, T.; Meurman, J.H. Periodontal microorganisms and diagnosis of malignancy: A cross-sectional study. Tumor Biol. 2021, 43, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Meurman, J.H.; Bascones-Martinez, A. Are oral and dental diseases linked to cancer? Oral. Dis. 2011, 17, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Bascones-Martínez, A.; García-García, V.; Meurman, J.H.; Requena-Caballero, L. Immune-mediated diseases: What can be found in the oral cavity? Int. J. Derm. 2015, 54, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Jenning, M.; Marklein, B.; Ytterberg, J.; Zubarev, R.A.; Joshua, V.; van Schaardenburg, D.; van de Stadt, L.; Catrina, A.I.; Nonhoff, U.; Häupl, T.; et al. Bacterial citrullinated epitopes generated by Porphyromonas gingivalis infection-a missing link for ACPA production. Ann. Rheum. Dis. 2020, 79, 1194–1202. [Google Scholar] [CrossRef]
- Julkunen, A.; Heikkinen, A.M.; Söder, B.; Söder, P.Ö.; Toppila-Salmi, S.; Meurman, J.H. Autoimmune diseases and oral health: 30-year follow-up of a Swedish cohort. Dent. J. 2017, 6, 1. [Google Scholar] [CrossRef] [Green Version]


| Database | Source |
|---|---|
| Socio-economic population register | Statistics Sweden (SCB) |
| Open ward (polyclinic) register | Swedish National Board of Health and Welfare |
| Hospital register | Swedish National Board of Health and Welfare |
| Dental treatment register | Swedish National Board of Health and Welfare |
| Prescription medicine register | Swedish National Board of Health and Welfare |
| Heart infarction register | Swedish National Board of Health and Welfare |
| Cancer register | Swedish National Board of Health and Welfare |
| Death register | Swedish National Board of Health and Welfare |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meurman, J.H.; Söder, B. The Stockholm Study: Over 30 years’ Observation of the Effect of Oral Infections on Systemic Health. Dent. J. 2022, 10, 68. https://doi.org/10.3390/dj10040068
Meurman JH, Söder B. The Stockholm Study: Over 30 years’ Observation of the Effect of Oral Infections on Systemic Health. Dentistry Journal. 2022; 10(4):68. https://doi.org/10.3390/dj10040068
Chicago/Turabian StyleMeurman, Jukka H., and Birgitta Söder. 2022. "The Stockholm Study: Over 30 years’ Observation of the Effect of Oral Infections on Systemic Health" Dentistry Journal 10, no. 4: 68. https://doi.org/10.3390/dj10040068
APA StyleMeurman, J. H., & Söder, B. (2022). The Stockholm Study: Over 30 years’ Observation of the Effect of Oral Infections on Systemic Health. Dentistry Journal, 10(4), 68. https://doi.org/10.3390/dj10040068