Effect of EDTA Gel on Residual Subgingival Calculus and Biofilm: An In Vitro Pilot Study
Abstract
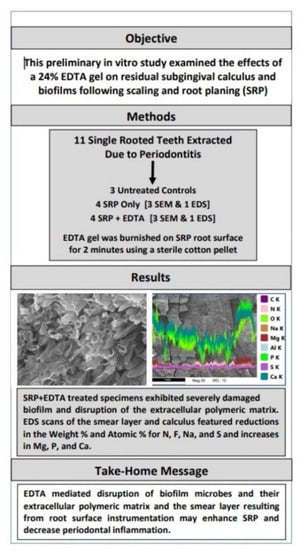
:1. Introduction
2. Materials and Methods
2.1. Specimens
2.2. Treatment
2.3. Scanning Electron Microscopy (SEM)
2.4. Energy-Dispersive X-ray Spectroscopy (EDS)
3. Results
3.1. SEM Examination
3.2. EDS Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Harrel, S.K.; Wilson, T.G., Jr.; Rivera-Hidalgo, F. A videoscope for use in minimally invasive periodontal surgery. J. Clin. Periodontol. 2013, 40, 868–874. [Google Scholar] [CrossRef] [Green Version]
- Harrel, S.K.; Valderrama, P.; Barnes, J.B.; Blackwell, E.L. Frequency of root surface microgrooves associated with periodontal destruction. Int. J. Periodontics Restor. Dent. 2016, 36, 841–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrel, S.K.; Wilson, T.G.; Tunnell, J.C.; Stenberg, W.V. Laser identification of residual microislands of calculus and their removal with chelation. J. Periodontol. 2020, 91, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Harrel, S.K.; Nunn, M.E.; Abraham, C.M.; Rivera-Hidalgo, F.; Shulman, J.D.; Tunnell, J.C. Videoscope assisted minimally invasive surgery (VMIS): 36-month results. J. Periodontol. 2017, 88, 528–535. [Google Scholar] [CrossRef]
- Mae, M.; Alam, M.I.; Yasunori, Y.; Ozaki, Y.; Higuchi, K.; Ziauddin, S.M.; Raudales, J.L.M.; Sakai, E.; Tsukuba, T.; Yoshimura, A. The role of cytokines produced via the NLRP3 inflammasome in mouse macrophages stimulated with dental calculus in osteoclastogenesis. Int. J. Mol. Sci. 2021, 22, 12434. [Google Scholar] [CrossRef] [PubMed]
- Lebre, F.; Sridharan, R.; Sawkins, M.J.; Kelly, D.J.; O’Brien, F.J.; Lavelle, E.C. The shape and size of hydroxyapatite particles dictate inflammatory responses following implantation. Sci. Rep. 2017, 7, 2922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziauddin, S.M.; Alam, M.I.; Mae, M.; Oohira, M.; Higuchi, K.; Yamashita, Y.; Ozaki, Y.; Yoshimura, A. Cytotoxic effects of dental calculus particles and freeze-dried Aggregatibacter actinomycetemcomitans and Fusobacterium nucleatum on HSC-2 oral epithelial cells and THP-1 macrophages. J. Periodontol. 2022, 93, e92–e103. [Google Scholar] [CrossRef]
- Cobb, C.M.; Sottosanti, J.S. A re-evaluation of scaling and root planing. J. Periodontol. 2021, 92, 1370–1378. [Google Scholar] [CrossRef]
- Rohanizadeh, R.; Legeros, R.Z. Ultrastructural study of calculus enamel and calculus-root interfaces. Arch. Oral Biol. 2005, 50, 89–96. [Google Scholar] [CrossRef]
- Aspriello, S.D.; Piemontese, M.; Levrini, L.; Sauro, S. Ultramorphology of the root surface subsequent to hand-ultrasonic simultaneous instrumentation during non-surgical periodontal treatments. An in vitro study. J. Appl. Oral Sci. 2011, 19, 74–81. [Google Scholar] [CrossRef]
- Morrison, S.L.; Cobb, C.M.; Kazakos, G.M.; Killoy, W.J. Root surface characteristics associated with subgingival placement of monolithic tetracycline-impregnated fibers. J. Periodontol. 1992, 63, 137–143. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Finnegan, S.; Percival, S.L. EDTA: An antimicrobial and antibiofilm agent for use in wound care. Adv. Wound Care 2015, 4, 415–421. [Google Scholar] [CrossRef] [Green Version]
- Walsh, S.E.; Maillard, J.Y.; Russell, A.D.; Catrenich, C.E.; Charbonneau, D.L.; Bartolo, R.G. Activity and mechanisms of action of selected biocidal agents on Gram-positive and—Negative bacteria. J. Appl. Microbiol. 2003, 94, 240–247. [Google Scholar] [CrossRef]
- Chang, Y.; Gu, W.; McLandsborough, L. Low concentration of ethylenediaminetetraacetic acid (EDTA) affects biofilm formation of Listeria monocytogenes by inhibiting its initial adherence. Food Microbiol. 2012, 29, 10–17. [Google Scholar] [CrossRef]
- Banin, E.; Brady, K.M.; Greenberg, E.P. Chelator-induced dispersal and killing of Pseudomonas aeruginosa cells in a biofilm. Appl. Environ. Microbiol. 2006, 72, 2064–2069. [Google Scholar] [CrossRef] [Green Version]
- Barootchi, S.; Tavelli, L.; Ravida, A.; Wang, C.-W.; Wang, H.-L. Effect of EDTA root conditioning on the outcome of coronally advanced flap with connective tissue graft: A systematic review and meta-analysis. Clin. Oral Investig. 2018, 22, 2727–2741. [Google Scholar] [CrossRef]
- Górski, B.; Szerszeń, M.; Kaczyński, T. Effect of 24% EDTA root conditioning on the outcome of modified coronally advanced tunnel technique with subepithelial connective tissue graft for the treatment of multiple gingival recessions: A randomized clinical trial. Clin. Oral Investig. 2022, 26, 1761–1772. [Google Scholar] [CrossRef]
- Blomlof, J.; Lindskog, S. Root surface texture and early cell and tissue colonization after different etching modalities. Eur. J. Oral Sci. 1995, 103, 17–24. [Google Scholar] [CrossRef]
- Gamal, A.Y.; Mailhot, J.M. The effects of EDTA gel conditioning exposure time on periodontitis affected human root surfaces: Surface topography and PDL cell adhesion. J. Int. Acad. Periodontol. 2003, 5, 11–22. [Google Scholar]
- Cohen, M.; Garnick, J.J.; Ringle, R.D.; Hanes, P.J.; Thompson, W.O. Calcium and phosphorus content of roots exposed to the oral environment. J. Clin. Periodontol. 1992, 19, 268–273. [Google Scholar] [CrossRef]
- Sakae, T.; Yamamoto, H.; Mishima, H.; Matsumoto, T.; Kozawa, Y. Morphology and chemical composition of dental calculi mainly composed of whitlockite. Scanning Microsc. 1989, 3, 855–860. [Google Scholar]
- Bosshardt, D.D.; Selvig, K.A. Dental cementum: The dynamic tissue covering of the root. Periodontology 2000 1997, 13, 41–75. [Google Scholar] [CrossRef]
- Atilla, G.; Baylas, H. Electron probe analysis of cementum surfaces. J. Marmara Univ. Dent. Fac. 1996, 2, 510–514. [Google Scholar]
- Atilla, G.; Baylas, H. Effect of various demineralizing agents on mineral contents of cementum surfaces (an electron probe analysis). J. Marmara Univ. Dent. Fac. 1996, 2, 515–519. [Google Scholar]
- Nasreldin, Z.M.; Awooda, E.M.; Hashim, N.T. Microscopic differences in cementum structure and mineral composition of teeth extracted from patients with gingivitis, chronic periodontitis and aggressive periodontitis. A preliminary comparative study. Int. J. Dent. Sci. Res. 2016, 4, 90–94. [Google Scholar]
- Colaco, E.; Brouri, D.; Méthivier, C.; Valentin, L.; Oudet, F.; El Kirat, K.; Guibert, C.; Landoulsi, J. Calcium phosphate mineralization through homogenous enzymatic catalysis: Investigation of the early stages. J. Colloid Interface Sci. 2020, 565, 43–54. [Google Scholar] [CrossRef]
- Shah, F.A. Magnesium whitlockite—Omnipresent in pathological mineralisation of soft tissues but not a significant inorganic constituent of bone. Acta Biomater. 2021, 125, 72–82. [Google Scholar] [CrossRef]
- Song, B.; Leff, L.G. Influence of magnesium ions on biofilm formation by Pseudomonas fluorescens. Microbiol. Res. 2006, 161, 355–361. [Google Scholar] [CrossRef]
- Wirthlin, M.R.; Pederson, E.D.; Hancock, E.B.; Lamberts, B.L.; Leonard, E.P. The hypermineralization of diseased root surfaces. J. Periodontol. 1979, 50, 125–127. [Google Scholar] [CrossRef]














Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cobb, C.M.; Harrel, S.K.; Zhao, D.; Spencer, P. Effect of EDTA Gel on Residual Subgingival Calculus and Biofilm: An In Vitro Pilot Study. Dent. J. 2023, 11, 22. https://doi.org/10.3390/dj11010022
Cobb CM, Harrel SK, Zhao D, Spencer P. Effect of EDTA Gel on Residual Subgingival Calculus and Biofilm: An In Vitro Pilot Study. Dentistry Journal. 2023; 11(1):22. https://doi.org/10.3390/dj11010022
Chicago/Turabian StyleCobb, Charles M., Stephen K. Harrel, Donggao Zhao, and Paulette Spencer. 2023. "Effect of EDTA Gel on Residual Subgingival Calculus and Biofilm: An In Vitro Pilot Study" Dentistry Journal 11, no. 1: 22. https://doi.org/10.3390/dj11010022
APA StyleCobb, C. M., Harrel, S. K., Zhao, D., & Spencer, P. (2023). Effect of EDTA Gel on Residual Subgingival Calculus and Biofilm: An In Vitro Pilot Study. Dentistry Journal, 11(1), 22. https://doi.org/10.3390/dj11010022