Clinical and Biological Validation of an Allogeneous Cancellous Bone Block for Alveolar Maxillary Ridge Reconstruction: A Case Series
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Series Setup
2.2. Patient Selection
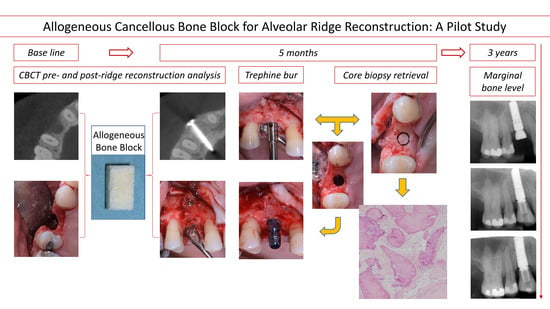
2.3. Bone Block Augmentation Procedure
2.4. Implant Placement and Core Biopsy Removal
2.5. Radiographic Measurements
2.6. Histology
2.7. Case Series Setup
2.7.1. Case 1
2.7.2. Case 2
2.7.3. Case 3
3. Results
3.1. Clinical Results
3.2. Radiometric Assessment
3.3. Histological Characterization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Araujo, M.G.; Sukekava, F.; Wennstrom, J.L.; Lindhe, J. Ridge Alterations Following Implant Placement in Fresh Extraction Sockets: An Experimental Study in the Dog. J. Clin. Periodontol. 2005, 32, 645–652. [Google Scholar] [CrossRef] [PubMed]
- McAllister, B.S.; Haghighat, K. Bone Augmentation Techniques. J. Periodontol. 2007, 78, 377–396. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Dula, K.; Hirt, H.P.; Schenk, R.K. Lateral Ridge Augmentation Using Autografts and Barrier Membranes: A Clinical Study with 40 Partially Edentulous Patients. J. Oral Maxillofac. Surg. 1996, 54, 420–432. [Google Scholar] [CrossRef]
- von Arx, T.; Buser, D. Horizontal Ridge Augmentation Using Autogenous Block Grafts and the Guided Bone Regeneration Technique with Collagen Membranes: A Clinical Study with 42 Patients. Clin. Oral Implant. Res. 2006, 17, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, R.S.; Sayar, F.; Rakhshan, V.; Iranpour, B.; Jahanbani, J.; Toumaj, A.; Akhoondi, N. Clinical and Histomorphometric Assessment of Lateral Alveolar Ridge Augmentation Using a Corticocancellous Freeze-Dried Allograft Bone Block. J. Oral Implantol. 2017, 43, 202–210. [Google Scholar] [CrossRef]
- Solakoğlu, Ö.; Ofluoğlu, D.; Schwarzenbach, H.; Heydecke, G.; Reißmann, D.; Ergun, S.; Götz, W. A 3-Year Prospective Randomized Clinical Trial of Alveolar Bone Crest Response and Clinical Parameters through 1, 2, and 3 Years of Clinical Function of Implants Placed 4 Months after Alveolar Ridge Preservation Using Two Different Allogeneic Bone-Grafting Materials. Int. J. Implant. Dent. 2022, 8, 5. [Google Scholar] [CrossRef]
- Elnayef, B.; Porta, C.; del Amo, F.; Mordini, L.; Gargallo-Albiol, J.; Hernández-Alfaro, F. The Fate of Lateral Ridge Augmentation: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implants 2018, 33, 622–635. [Google Scholar] [CrossRef]
- Troeltzsch, M.; Troeltzsch, M.; Kauffmann, P.; Gruber, R.; Brockmeyer, P.; Moser, N.; Rau, A.; Schliephake, H. Clinical Efficacy of Grafting Materials in Alveolar Ridge Augmentation: A Systematic Review. J. Cranio-Maxillofac. Surg. 2016, 44, 1618–1629. [Google Scholar] [CrossRef]
- Chavda, S.; Levin, L. Human Studies of Vertical and Horizontal Alveolar Ridge Augmentation Comparing Different Types of Bone Graft Materials: A Systematic Review. J. Oral Implantol. 2018, 44, 74–84. [Google Scholar] [CrossRef]
- Keith, J.D. Localized Ridge Augmentation with a Block Allograft Followed by Secondary Implant Placement: A Case Report. Int. J. Periodontics Restor. Dent. 2004, 24, 11–17. [Google Scholar]
- Wallace, S.; Gellin, R. Clinical Evaluation of Freeze-Dried Cancellous Block Allografts for Ridge Augmentation and Implant Placement in the Maxilla. Implant. Dent. 2010, 19, 272–279. [Google Scholar] [CrossRef]
- Sanz, M.; Dahlin, C.; Apatzidou, D.; Artzi, Z.; Bozic, D.; Calciolari, E.; De Bruyn, H.; Dommisch, H.; Donos, N.; Eickholz, P.; et al. Biomaterials and Regenerative Technologies Used in Bone Regeneration in the Craniomaxillofacial Region: Consensus Report of Group 2 of the 15th European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46, 82–91. [Google Scholar] [CrossRef]
- Pérez-González, F.; Molinero-Mourelle, P.; Sánchez-Labrador, L.; Sáez-Alcaide, L.; Limones, A.; Cortés-Bretón Brinkmann, J.; López-Quiles, J. Assessment of Clinical Outcomes and Histomorphometric Findings in Alveolar Ridge Augmentation Procedures with Allogeneic Bone Block Grafts: A Systematic Review and Meta-Analysis. Med. Oral 2020, 25, E291–E298. [Google Scholar] [CrossRef]
- Monje, A.; Pikos, M.A.; Chan, H.-L.; Suarez, F.; Gargallo-Albiol, J.; Hernández-Alfaro, F.; Galindo-Moreno, P.; Wang, H.-L. On the Feasibility of Utilizing Allogeneic Bone Blocks for Atrophic Maxillary Augmentation. BioMed Res. Int. 2014, 2014, 814578. [Google Scholar] [CrossRef] [PubMed]
- Starch-Jensen, T.; Aludden, H.; Hallman, M.; Dahlin, C.; Christensen, A.-E.; Mordenfeld, A. A Systematic Review and Meta-Analysis of Long-Term Studies (Five or More Years) Assessing Maxillary Sinus Floor Augmentation. Int. J. Oral. Maxillofac. Surg. 2018, 47, 103–116. [Google Scholar] [CrossRef]
- Kappe, T.; Cakir, B.; Mattes, T.; Reichel, H.; Flören, M. Infections after Bone Allograft Surgery: A Prospective Study by a Hospital Bone Bank Using Frozen Femoral Heads from Living Donors. Cell Tissue Bank. 2010, 11, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Fretwurst, T.; Spanou, A.; Nelson, K.; Wein, M.; Steinberg, T.; Stricker, A. Comparison of Four Different Allogeneic Bone Grafts for Alveolar Ridge Reconstruction: A Preliminary Histologic and Biochemical Analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, 424–431. [Google Scholar] [CrossRef]
- Ghanaati, S.; Barbeck, M.; Booms, P.; Lorenz, J.; Kirkpatrick, C.J.; Sader, R.A. Potential Lack of “Standardized” Processing Techniques for Production of Allogeneic and Xenogeneic Bone Blocks for Application in Humans. Acta Biomater. 2014, 10, 3557–3562. [Google Scholar] [CrossRef]
- Lorenz, J.; Kubesch, A.; Al-Maawi, S.; Schwarz, F.; Sader, R.A.; Schlee, M.; Ghanaati, S. Allogeneic Bone Block for Challenging Augmentation—A Clinical, Histological, and Histomorphometrical Investigation of Tissue Reaction and New Bone Formation. Clin. Oral Investig. 2018, 22, 3159–3169. [Google Scholar] [CrossRef]
- Lorenz, J.; Schlee, M.; Al-Maawi, S.; Chia, P.; Sader, R.; Ghanaati, S. Variant Purification of an Allogeneic Bone Block. Acta Stomatol. Croat. 2017, 51, 141–147. [Google Scholar] [CrossRef]
- Blume, O.; Donkiewicz, P.; Palkovics, D.; Götz, W.; Windisch, P. Volumetric Changes of a Customized Allogeneic Bone Block Measured by Two Image Matching Tools: Introduction of a Novel Assessment Technique for Graft Resorption. Acta Stomatol. Croat. 2021, 55, 406–417. [Google Scholar] [CrossRef]
- Otto, S.; Kleye, C.; Burian, E.; Ehrenfeld, M.; Cornelius, C.-P. Custom-Milled Individual Allogeneic Bone Grafts for Alveolar Cleft Osteoplasty—A Technical Note. J. Cranio-Maxillofac. Surg. 2017, 45, 1955–1961. [Google Scholar] [CrossRef] [PubMed]
- Solakoglu, Ö.; Götz, W.; Heydecke, G.; Schwarzenbach, H. Histological and Immunohistochemical Comparison of Two Different Allogeneic Bone Grafting Materials for Alveolar Ridge Reconstruction: A Prospective Randomized Trial in Humans. Clin. Implant. Dent. Relat. Res. 2019, 21, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- Keane, T.J.; Londono, R.; Turner, N.J.; Badylak, S.F. Consequences of Ineffective Decellularization of Biologic Scaffolds on the Host Response. Biomaterials 2012, 33, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.N.; Valentin, J.E.; Stewart-Akers, A.M.; McCabe, G.P.; Badylak, S.F. Macrophage Phenotype and Remodeling Outcomes in Response to Biologic Scaffolds with and without a Cellular Component. Biomaterials 2009, 30, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Chappard, D.; Fressonnet, C.; Geni, C. Fat in Bone Xenografts: Importance of the Purification Procedures on Cleanliness, Wettability and Biocompatibility. Biomaterials 1993, 14, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Puišys, A.; Žukauskas, S.; Kubilius, R.; Vindašiūtė, E.; Linkevičius, T. Bone Augmentation and Simultaneous Soft Tissue Thickening with Collagen Tissue Matrix Derivate Membrane in an Aesthetic Area. A Case Report. Stomatologija 2017, 19, 5. [Google Scholar]
- Nissan, J.; Ghelfan, O.; Mardinger, O.; Calderon, S.; Chaushu, G. Efficacy of Cancellous Block Allograft Augmentation Prior to Implant Placement in the Posterior Atrophic Mandible: Cancellous Block Allografts in the Posterior Atrophic Mandible. Clin. Implant. Dent. Relat. Res. 2011, 13, 279–285. [Google Scholar] [CrossRef]
- Nissan, J.; Gross, O.; Mardinger, O.; Ghelfan, O.; Sacco, R.; Chaushu, G. Post-Traumatic Implant-Supported Restoration of the Anterior Maxillary Teeth Using Cancellous Bone Block Allografts. J. Oral Maxillofac. Surg. 2011, 69, e513–e518. [Google Scholar] [CrossRef]
- Nissan, J.; Mardinger, O.; Calderon, S.; Romanos, G.E.; Chaushu, G. Cancellous Bone Block Allografts for the Augmentation of the Anterior Atrophic Maxilla: Cancellous Block Allograft in the Anterior Atrophic Maxilla. Clin. Implant. Dent. Relat. Res. 2011, 13, 104–111. [Google Scholar] [CrossRef]
- Toscano, N.; Holtzclaw, D.; Mazor, Z.; Rosen, P.; Horowitz, R.; Toffler, M. Horizontal Ridge Augmentation Utilizing a Composite Graft of Demineralized Freeze-Dried Allograft, Mineralized Cortical Cancellous Chips, and a Biologically Degradable Thermoplastic Carrier Combined with a Resorbable Membrane: A Retrospective Evaluation of 73 Consecutively Treated Cases from Private Practices. J. Oral Implantol. 2010, 36, 467–474. [Google Scholar] [CrossRef]
- Tresguerres, F.G.F.; Cortes, A.R.G.; Vallejo, G.H.; Cabrejos-Azama, J.; Tamimi, F.; Torres, J. Clinical and Radiographic Outcomes of Allogeneic Block Grafts for Maxillary Lateral Ridge Augmentation: A Randomized Clinical Trial. Clin. Implant. Dent. Relat. Res. 2019, 21, 1087–1098. [Google Scholar] [CrossRef]
- Nissan, J.; Marilena, V.; Gross, O.; Mardinger, O.; Chaushu, G. Histomorphometric Analysis Following Augmentation of the Anterior Atrophic Maxilla with Cancellous Bone Block Allograft. Int. J. Oral Maxillofac. Implant. 2012, 27, 84–89. [Google Scholar]
- Lorenz, J.; Kubesch, A.; Korzinskas, T.; Barbeck, M.; Landes, C.; Sader, R.A.; Kirkpatrick, C.J.; Ghanaati, S. TRAP-Positive Multinucleated Giant Cells Are Foreign Body Giant Cells Rather Than Osteoclasts: Results from a Split-Mouth Study in Humans. J. Oral Implantol. 2015, 41, e257–e266. [Google Scholar] [CrossRef] [PubMed]
- Chaushu, G.; Mardinger, O.; Calderon, S.; Moses, O.; Nissan, J. The Use of Cancellous Block Allograft for Sinus Floor Augmentation with Simultaneous Implant Placement in the Posterior Atrophic Maxilla. J. Periodontol. 2009, 80, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Chaushu, L.; Chaushu, G.; Kolerman, R.; Vered, M.; Naishlos, S.; Nissan, J. Histomorphometrical Assessment of Sinus Augmentation Using Allograft (Particles or Block) and Simultaneous Implant Placement. Sci. Rep. 2020, 10, 9046. [Google Scholar] [CrossRef] [PubMed]
- Ersanli, S.; Olgac, V.; Leblebicioglu, B. Histologic Analysis of Alveolar Bone Following Guided Bone Regeneration. J. Periodontol. 2004, 75, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Wu, Y.; Huang, W.; Zhou, W.; Galindo-Moreno, P.; Montanero-Fernandez, J.; Sheridan, R.; Wang, H.-L.; Wang, F. Influence of Posterior Mandibular Dimensions on Alveolar Bone Microarchitecture. Int. J. Oral Maxillofac. Implant. 2017, 32, 423–430. [Google Scholar] [CrossRef]
- Sanz-Sánchez, I.; Ortiz-Vigón, A.; Sanz-Martín, I.; Figuero, E.; Sanz, M. Effectiveness of Lateral Bone Augmentation on the Alveolar Crest Dimension: A Systematic Review and Meta-Analysis. J. Dent. Res. 2015, 94, 128S–142S. [Google Scholar] [CrossRef]
- Sanz-Sánchez, I.; Carrillo De Albornoz, A.; Figuero, E.; Schwarz, F.; Jung, R.; Sanz, M.; Thoma, D. Effects of Lateral Bone Augmentation Procedures on Peri-implant Health or Disease: A Systematic Review and Meta-analysis. Clin. Oral Implant. Res. 2018, 29, 18–31. [Google Scholar] [CrossRef]
- Smeets, R.; Matthies, L.; Windisch, P.; Gosau, M.; Jung, R.; Brodala, N.; Stefanini, M.; Kleinheinz, J.; Payer, M.; Henningsen, A.; et al. Horizontal Augmentation Techniques in the Mandible: A Systematic Review. Int. J. Implant. Dent. 2022, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Gorgis, R.; Qazo, L.; Bruun, N.H.; Starch-Jensen, T. Lateral Alveolar Ridge Augmentation with an Autogenous Bone Block Graft Alone with or without Barrier Membrane Coverage: A Systematic Review and Meta-Analysis. J. Oral Maxillofac. Res. 2021, 12, e1. [Google Scholar] [CrossRef] [PubMed]
- Venet, L.; Perriat, M.; Mangano, F.G.; Fortin, T. Horizontal Ridge Reconstruction of the Anterior Maxilla Using Customized Allogeneic Bone Blocks with a Minimally Invasive Technique—A Case Series. BMC Oral Health 2017, 17, 146. [Google Scholar] [CrossRef] [PubMed]











| Patient | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Gender | F | M | M | M | F |
| Age | 54 | 53 | 68 | 63 | 62 |
| Treatment positions | 14 | 13, 14 | 27 | 24, 26 | 14, 16 |
| Implant Type | BL RC | BL RC | TL WN | BL RC | TL WN |
| Implant Dimensions | Ø4.1 × 12 mm | Ø4.1 × 10 mm Ø4.1 × 10 mm | Ø4.8 × 10 mm | Ø4.1 × 10 mm Ø4.8 × 10 mm | Ø4.1 × 10 mm Ø4.8 × 10 mm |
| Insertion Torque [N/cm] | 45 | 35, 40 | 30 | 40, 32 | 35, 30 |
| Patient | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 †,§ | 3 | 4 † | 5 †,†† | Median (1st to 3rd IQR | Range | |
| Horizontal Dimensions (CBCT) [mm] | |||||||
| Baseline | 3.0 | 2.0 | 2.0 | 5.0, 8.4 | 3.0 | 3.0 (2.3 to 4.5) | 2.0–8.4 |
| 5-month follow-up | 8.0 | 10 | 11.5 | 12.0, 12.5 | 10 | 10.8 (10 to 11.9) | 8.0–12.5 |
| Horizontal Gain ‡ | 5.0(×9.0) ‡ | 8.0(×9.0) | 9.5(×8.0) | 7.0 (×11.0), 4.0 (×11.0) | 7.0(×5.2) | 7.0 (5.5 to 7.8) | 4.0–9.5 |
| Marginal Bone Level (2D) [mm] | |||||||
| Post-OP mesial | 0.0 | 0.0, 0.3 | 0.2 | 0.0, 0.0 | 0.2 | 0.0 (0.0 to 0.2) | 0.0–0.3 |
| 1-year mesial | −0.1 | 0.0, 0.3 | 0.0 | 0.0, −1.2 | 0.0 | 0.0 (−0.1 to 0.0) | −1.2–0.3 |
| Post-OP distal | 0.0 | 0.3, 0.0 | 0.0 | 0.0, 0.0 | 0.0 | 0.0 (0.0 to 0.0) | 0.0–0.3 |
| 1-year distal | 0.0 | 0.3, −0.0 | 0.0 | 0.0, −1.2 | 0.0 | 0.0 (0.0 to 0.0) | −1.2–0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez, A.; Pierantozzi, E.; Di Felice, R.; Lombardi, T. Clinical and Biological Validation of an Allogeneous Cancellous Bone Block for Alveolar Maxillary Ridge Reconstruction: A Case Series. Dent. J. 2024, 12, 42. https://doi.org/10.3390/dj12020042
Perez A, Pierantozzi E, Di Felice R, Lombardi T. Clinical and Biological Validation of an Allogeneous Cancellous Bone Block for Alveolar Maxillary Ridge Reconstruction: A Case Series. Dentistry Journal. 2024; 12(2):42. https://doi.org/10.3390/dj12020042
Chicago/Turabian StylePerez, Alexandre, Elena Pierantozzi, Roberto Di Felice, and Tommaso Lombardi. 2024. "Clinical and Biological Validation of an Allogeneous Cancellous Bone Block for Alveolar Maxillary Ridge Reconstruction: A Case Series" Dentistry Journal 12, no. 2: 42. https://doi.org/10.3390/dj12020042
APA StylePerez, A., Pierantozzi, E., Di Felice, R., & Lombardi, T. (2024). Clinical and Biological Validation of an Allogeneous Cancellous Bone Block for Alveolar Maxillary Ridge Reconstruction: A Case Series. Dentistry Journal, 12(2), 42. https://doi.org/10.3390/dj12020042