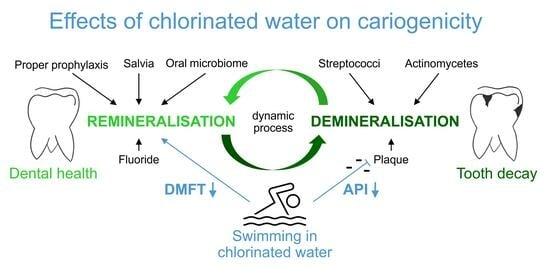
Dental Health Benefits of Swimming in Chlorinated Water
Abstract
:1. Introduction
2. Materials and Methods
- <5%—optimal oral hygiene;
- 5–35%—good oral hygiene, improvement possible;
- 35–50%—oral hygiene needs improvement;
- 50–70%—poor oral hygiene;
- >70%—inadequate oral hygiene.
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hescot, P. The New Definition of Oral Health and Relationship between Oral Health and Quality of Life. Chin. J. Dent. Res. 2017, 20, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The oral microbiome: Role of key organisms and complex networks in oral health and disease. Periodontology 2000 2021, 87, 107–131. [Google Scholar] [CrossRef]
- Carrizales-Sepúlveda, E.F.; Ordaz-Farías, A.; Vera-Pineda, R.; Flores-Ramírez, R. Periodontal Disease, Systemic Inflammation and the Risk of Cardiovascular Disease. Heart Lung Circ. 2018, 27, 1327–1334. [Google Scholar] [CrossRef]
- Sanderink, R.B.A.; Renggli, H.H.; Saxer, U.P. Orale Präventivmedizin; Georg Thieme Verlag KG: Stuttgart, Germany, 2022; ISBN 9783132051812. [Google Scholar]
- Mathur, V.P.; Dhillon, J.K. Dental Caries: A Disease Which Needs Attention. Indian J. Pediatr. 2018, 85, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Micheelis, W. (Eds.) Fünfte Deutsche Mundgesundheitsstudie-(DMS IV); Deutscher Zahnärzte Verlag DÄV: Köln, Germany, 2016; ISBN 978-3-7691-0020-4. [Google Scholar]
- Bourgeois, D.M.; Llodra, J.C. Global burden of dental condition among children in nine countries participating in an international oral health promotion programme, 2012–2013. Int. Dent. J. 2014, 64 (Suppl. 2), 27–34. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- D’Ercole, S.; Tieri, M.; Martinelli, D.; Tripodi, D. The effect of swimming on oral health status: Competitive versus non-competitive athletes. J. Appl. Oral Sci. 2016, 24, 107–113. [Google Scholar] [CrossRef]
- Lazar, J.M.; Khanna, N.; Chesler, R.; Salciccioli, L. Swimming and the heart. Int. J. Cardiol. 2013, 168, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Rathee, M.; Sapra, A. StatPearls: Dental Caries; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Needleman, I.; Ashley, P.; Petrie, A.; Fortune, F.; Turner, W.; Jones, J.; Niggli, J.; Engebretsen, L.; Budgett, R.; Donos, N.; et al. Oral health and impact on performance of athletes participating in the London 2012 Olympic Games: A cross-sectional study. Br. J. Sports Med. 2013, 47, 1054–1058. [Google Scholar] [CrossRef]
- Merle, C.L.; Richter, L.; Challakh, N.; Haak, R.; Schmalz, G.; Needleman, I.; Wolfarth, B.; Ziebolz, D.; Wüstenfeld, J. Orofacial conditions and oral health behavior of young athletes: A comparison of amateur and competitive sports. Scand. J. Med. Sci. Sports 2022, 32, 903–912. [Google Scholar] [CrossRef]
- Bryant, S.; McLaughlin, K.; Morgaine, K.; Drummond, B. Elite athletes and oral health. Int. J. Sports Med. 2011, 32, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, D.; Cosi, A.; Fulco, D.; D’Ercole, S. The Impact of Sport Training on Oral Health in Athletes. Dent. J. 2021, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Rateitschak-Plüss, E.M.; Rateitschak-Plüss, E. Band 1: Parodontologie, 3.; vollständig überarbeitete und erweiterte Auflage; Thieme: Stuttgart, Germany, 2003; ISBN 3-13-655603-8. [Google Scholar]
- Gudipaneni, R.K.; Alkuwaykibi, A.S.; Ganji, K.K.; Bandela, V.; Karobari, M.I.; Hsiao, C.-Y.; Kulkarni, S.; Thambar, S. Assessment of caries diagnostic thresholds of DMFT, ICDAS II and CAST in the estimation of caries prevalence rate in first permanent molars in early permanent dentition—A cross-sectional study. BMC Oral Health 2022, 22, 133. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.Z.R.; Cepeda, M.A.A.N.; Lopez-Martinez, F.; Arana, H.L.M.; Ocampo, J.C.A.; Ceniceros, F.G.A.; Urbina, I.N.V.; Soto, J.M.S. A comparison of DMFT, ICDAS II and CAST. Int. J. Appl. Dent. Sci. 2023, 9, 231–235. [Google Scholar] [CrossRef]
- Needleman, I.; Ashley, P.; Fine, P.; Haddad, F.; Loosemore, M.; de Medici, A.; Donos, N.; Newton, T.; van Someren, K.; Moazzez, R.; et al. Oral health and elite sport performance. Br. J. Sports Med. 2015, 49, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Ashley, P.; Di Iorio, A.; Cole, E.; Tanday, A.; Needleman, I. Oral health of elite athletes and association with performance: A systematic review. Br. J. Sports Med. 2015, 49, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Frese, C.; Frese, F.; Kuhlmann, S.; Saure, D.; Reljic, D.; Staehle, H.J.; Wolff, D. Effect of endurance training on dental erosion, caries, and saliva. Scand. J. Med. Sci. Sports 2015, 25, e319–e326. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Qadir, A.; Trakman, G.; Aziz, T.; Khattak, M.I.; Nabi, G.; Alharbi, M.; Alshammari, A.; Shahzad, M. Sports and Energy Drink Consumption, Oral Health Problems and Performance Impact among Elite Athletes. Nutrients 2022, 14, 5089. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Dash, P.; Jha, K.; Singh, A. An insight into the world of sports dentistry. J. Sports Med. Phys. Fit. 2021, 61, 1555–1561. [Google Scholar] [CrossRef]
- Ehrenfeld, M.; Gängler, P.; Hoffmann, T.; Schwenzer, N.; Willershausen, B. (Eds.) Konservierende Zahnheilkunde und Parodontologie; 3; Unveränderte Auflage; Thieme: Stuttgart, Germany, 2010; ISBN 9783131540737. [Google Scholar]
- Faustova, M.O.; Ananieva, M.M.; Basarab, Y.O.; Dobrobolska, O.V.; Vovk, I.M.; Loban’, G.A. Bacterial factors of cariogenicity (literature review). Wiad. Lek. 2018, 71, 378–382. [Google Scholar]
- Rugg-Gunn, A. Dental caries: Strategies to control this preventable disease. Acta Med. Acad. 2013, 42, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Arweiler, N.B.; Netuschil, L. The Oral Microbiota. Adv. Exp. Med. Biol. 2016, 902, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J. Detection and diagnosis of the early caries lesion. BMC Oral Health 2015, 15 (Suppl. 1), S3. [Google Scholar] [CrossRef] [PubMed]
- Garthe, I.; Maughan, R.J. Athletes and Supplements: Prevalence and Perspectives. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 126–138. [Google Scholar] [CrossRef] [PubMed]
- van der Sluijs, E.; Slot, D.E.; Hennequin-Hoenderdos, N.L.; van Leeuwen, M.; van der Weijden, G.A. Prebrushing rinse with water on plaque removal: A split-mouth design. Int. J. Dent. Hyg. 2017, 15, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Rossato, M.B.; Unfer, B.; May, L.G.; Braun, K.O. Analysis of the Effectiveness of Different Hygiene Procedures Used in Dental Prostheses. Oral Health Prev. Dent. 2011, 9, 221–227. [Google Scholar] [CrossRef]
- van der Weijden, F.A.; van der Sluijs, E.; Ciancio, S.G.; Slot, D.E. Can Chemical Mouthwash Agents Achieve Plaque/Gingivitis Control? Dent. Clin. 2015, 59, 799–829. [Google Scholar] [CrossRef] [PubMed]
- Buczkowska-Radlińska, J.; Łagocka, R.; Kaczmarek, W.; Górski, M.; Nowicka, A. Prevalence of dental erosion in adolescent competitive swimmers exposed to gas-chlorinated swimming pool water. Clin. Oral Investig. 2013, 17, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, L.; Pigliacelli, S.; Kerr, A.R. Severe and rapid erosion of dental enamel from swimming: A clinical report. J. Prosthet. Dent. 2011, 106, 219–223. [Google Scholar] [CrossRef]
- Moore, A.B.; Calleros, C.; Aboytes, D.B.; Myers, O.B. An assessment of chlorine stain and collegiate swimmers. Can. J. Dent. Hyg. 2019, 53, 166–171. [Google Scholar]
- Escartin, J.L.; Arnedo, A.; Pinto, V.; Vela, M.J. A study of dental staining among competitive swimmers. Community Dent. Oral Epidemiol. 2000, 28, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.A.; Thomas, S.; Kumar, J.K.; Narayan, V. Prevalence of Dentinal Hypersensitivity and Dental Erosion among Competitive Swimmers, Kerala, India. Indian J. Community Med. 2019, 44, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, M.S.; Su, S.; Crespo, C.J.; Hung, M. Men and Oral Health: A Review of Sex and Gender Differences. Am. J. Mens. Health 2021, 15, 15579883211016361. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Sohn, W.; Burt, B.A.; Sandretto, A.M.; Kolker, J.L.; Marshall, T.A.; Ismail, A.I. Cariogenicity of soft drinks, milk and fruit juice in low-income african-american children: A longitudinal study. J. Am. Dent. Assoc. 2008, 139, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Needleman, I.; Ashley, P.; Fairbrother, T.; Fine, P.; Gallagher, J.; Kings, D.; Maughan, R.J.; Melin, A.K.; Naylor, M. Nutrition and oral health in sport: Time for action. Br. J. Sports Med. 2018, 52, 1483–1484. [Google Scholar] [CrossRef]
- Needleman, I.; Rankin, A.; Ashley, P.; Fairbrother, T.; Fine, P.; Gallagher, J.; Kings, D.; Maughan, R.J.; Melin, A.K.; Naylor, M. Infographic. Nutrition and oral health in sport: Time for action. Br. J. Sports Med. 2019, 53, 1432–1433. [Google Scholar] [CrossRef]



| Swimmers n = 101 | Non-Swimmers n = 100 | p Value | |
|---|---|---|---|
| Patient characteristics * | |||
| Age (years) | 15 (14–17) | 18 (16–21) | <0.001 |
| % Female | 54 (54%) | 17 (17%) | <0.001 |
| BMI (kg/m2) | 20.1 (19.1–22.1) | 21.9 (19.7–23.4) | 0.004 |
| Training per Week (hours) | 12 (8–14) | 9 (7–14) | 0.11 |
| Higher education of parent | 44 (44%) | 45 (45%) | 0.84 |
| Eating habits | |||
| Sweets (never) | 47 (47%) | 55 (55%) | 0.38 |
| Sweets (once daily) | 40 (40%) | 36 (36%) | |
| Sweets (multiple daily) | 14 (14%) | 9 (9%) | |
| >3 daily meals | 47 (47%) | 44 (44%) | 0.72 |
| Daily juice intake | 9 (9%) | 24 (24%) | 0.004 |
| Oral hygiene habits | |||
| Daily flossing | 55 (55%) | 45 (45%) | 0.18 |
| Teeth brushing (weekly) | 14 (14–14) | 14 (14–14) | 0.05 |
| (a) | ||
|---|---|---|
| exp. Β (95% CI) | p Value | |
| a Univariable Model | 1.60 (1.11–2.31) | 0.01 |
| b Multivariable Model | 1.67 (1.06–2.65) | 0.03 1 |
| (b) | ||
| exp. Β (95% CI) | p Value | |
| a Univariable Model | 2.91 (1.69–2.71) | <0.001 |
| b Multivariable Model | 2.47 (2.08–2.94) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaugeler, B.S.; van der Stouwe, J.G.; Templin, C.; Schmied, C.M.; Lanzer, M.; Niederseer, D. Dental Health Benefits of Swimming in Chlorinated Water. Dent. J. 2024, 12, 87. https://doi.org/10.3390/dj12040087
Gaugeler BS, van der Stouwe JG, Templin C, Schmied CM, Lanzer M, Niederseer D. Dental Health Benefits of Swimming in Chlorinated Water. Dentistry Journal. 2024; 12(4):87. https://doi.org/10.3390/dj12040087
Chicago/Turabian StyleGaugeler, Barbara Sophie, Jan Gerrit van der Stouwe, Christian Templin, Christian M. Schmied, Martin Lanzer, and David Niederseer. 2024. "Dental Health Benefits of Swimming in Chlorinated Water" Dentistry Journal 12, no. 4: 87. https://doi.org/10.3390/dj12040087
APA StyleGaugeler, B. S., van der Stouwe, J. G., Templin, C., Schmied, C. M., Lanzer, M., & Niederseer, D. (2024). Dental Health Benefits of Swimming in Chlorinated Water. Dentistry Journal, 12(4), 87. https://doi.org/10.3390/dj12040087