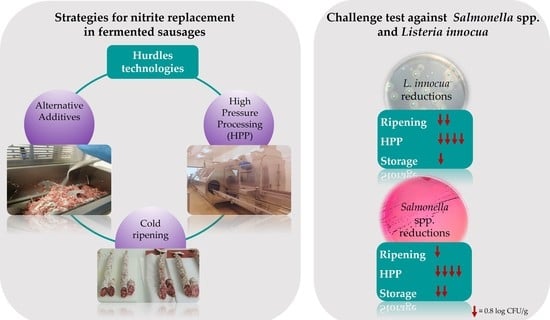
Strategies for Nitrite Replacement in Fermented Sausages and Effect of High Pressure Processing against Salmonella spp. and Listeria innocua
Abstract
:1. Introduction
2. Materials and Methods
2.1. Manufacturing of Fermented Sausages with Different Additives
2.2. Microbiological Analysis, pH, and Water Activity
2.3. Sensory Study
2.4. Challenge Test
2.4.1. Preparation of Inoculums
2.4.2. Production of Nitrite-Free Fermented Sausages and HPP Treatment
3. Results and Discussion
3.1. Effect of Additives and Ripening Conditions on Fermented Sausages Quality
3.1.1. Microbiological and Physicochemical Analysis
3.1.2. Sensory Study
3.2. Effect of Ripening, HPP, and Storage on L. innocua and Salmonella spp. Growth in Nitrite-Free Fermented Sausages
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cammack, R.; Joannou, C.L.; Cui, X.Y.; Torres Martinez, C.; Maraj, S.R.; Hughes, M.N. Nitrite and nitrosyl compounds in food preservation. Biochim. Biophys. Acta Bioenerg. 1999, 1411, 475–488. [Google Scholar] [CrossRef] [Green Version]
- Honikel, K.O. The use and control of nitrate and nitrite for the processing of meat products. Meat Sci. 2008, 78, 68–76. [Google Scholar] [CrossRef] [PubMed]
- EFSA; ECDC. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2015. EFSA J. 2016, 14, 20449. [Google Scholar] [CrossRef]
- Hospital, X.F.; Hierro, E.; Arnau, J.; Carballo, J.; Aguirre, J.S.; Gratacós-Cubarsí, M.; Fernández, M. Effect of nitrate and nitrite on Listeria and selected spoilage bacteria inoculated in dry-cured ham. Food Res. Int. 2017, 101, 82–87. [Google Scholar] [CrossRef]
- Christieans, S.; Picgirard, L.; Parafita, E.; Lebert, A.; Gregori, T. Impact of reducing nitrate/nitrite levels on the behavior of Salmonella Typhimurium and Listeria monocytogenes in French dry fermented sausages. Meat Sci. 2018, 137, 160–167. [Google Scholar] [CrossRef]
- Hospital, X.F.; Hierro, E.; Fernández, M. Effect of reducing nitrate and nitrite added to dry fermented sausages on the survival of Salmonella Typhimurium. Food Res. Int. 2014, 62, 410–415. [Google Scholar] [CrossRef]
- Reig, M.; Toldrá, F. Residues of harmful chemicals and their detection techniques. In Meat Quality Analysis Advanced Evaluation Methods, Techniques, and Technologies, 1st ed.; Biswas, A.K., Mandal, P., Eds.; Academic Press (Elsevier): London, UK, 2020; pp. 173–183. [Google Scholar] [CrossRef]
- Alirezalu, K.; Hesari, J.; Nemati, Z.; Munekata, P.E.S.; Barba, F.J.; Lorenzo, J.M. Combined effect of natural antioxidants and antimicrobial compounds during refrigerated storage of nitrite-free frankfurter-type sausage. Food Res. Int. 2019, 120, 839–850. [Google Scholar] [CrossRef]
- EC 1129/2011 European Commission regulation (EU) n° 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) n° 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives. Off. J. Eur. Union 2011, 295, 1–177.
- EC 780/2006 European Commission regulation (EU) n° 780/2006 amending Annex VI to Council Regulation (EEC) No 2092/91 on organic production of agricultural products and indications referring thereto on agricultural products and foodstuffs. Off. J. Eur. Union 2006, 137, 9–14.
- Sebranek, J.G.; Jackson-Davis, A.L.; Myers, K.L.; Lavieri, N.A. Beyond celery and starter culture: Advances in natural/organic curing processes in the United States. Meat Sci. 2012, 92, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Augusto, P.E.D.; Soares, B.M.C.; Castanha, N. Conventional Technologies of Food Preservation; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128110324. [Google Scholar]
- Leistner, L. Basic aspects of food preservation by hurdle technology. Int. J. Food Microbiol. 2000, 55, 181–186. [Google Scholar] [CrossRef]
- Italian Ministry of Health, Determinazione di L. monocytogenes e Salmonella spp. nei Prodotti a Base di Carne Suina Destinati All’export negli USA—Criteri e Modalità di Gestione Dell’autocontrollo Aziendale e Modalità di Verifica Dell’autorità Competente. 2015. Available online: https://www.aslmn.net/docs_file_vet/Circ_MdS_16_09_2015_Listeria_Salmonella_suino_export_USA.pdf (accessed on 30 August 2021).
- Bajovic, B.; Bolumar, T.; Heinz, V. Quality considerations with high pressure processing of fresh and value added meat products. Meat Sci. 2012, 92, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Garriga, M.; Marcos, B.; Martín, B.; Veciana-Nogués, M.T.; Bover-Cid, S.; Hugas, M.; Aymerich, T. Starter cultures and high-pressure processing to improve the hygiene and safety of slightly fermented sausages. J. Food Prot. 2005, 68, 2341–2348. [Google Scholar] [CrossRef]
- De Oliveira, T.L.C.; Ramos, A.L.S.; Ramos, E.M.; Piccoli, R.H.; Cristianini, M. Natural antimicrobials as additional hurdles to preservation of foods by high pressure processing. Trends Food Sci. Technol. 2015, 45, 60–85. [Google Scholar] [CrossRef]
- Huang, H.W.; Hsu, C.P.; Wang, C.Y. Healthy expectations of high hydrostatic pressure treatment in food processing industry. J. Food Drug Anal. 2020, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Koubaa, M.; do Prado-Silva, L.; Orlien, V.; de Souza Sant’Ana, A. Mild processing applied to the inactivation of the main foodborne bacterial pathogens: A review. Trends Food Sci. Technol. 2017, 66, 20–35. [Google Scholar] [CrossRef]
- Meloni, D. High-Hydrostatic-Pressure (HHP) Processing Technology as a Novel Control Method for Listeria monocytogenes Occurrence in Mediterranean-Style Dry-Fermented Sausages. Foods 2019, 8, 672. [Google Scholar] [CrossRef] [Green Version]
- Roohinejad, S.; Koubaa, M.; Sant’Ana, A.S.; Greiner, R. Mechanisms of Microbial Inactivation by Emerging Technologies; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128110324. [Google Scholar]
- Marcos, B.; Aymerich, T.; Dolors Guardia, M.; Garriga, M. Assessment of high hydrostatic pressure and starter culture on the quality properties of low-acid fermented sausages. Meat Sci. 2007, 76, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Rubio, B.; Possas, A.; Rincón, F.; García-Gímeno, R.M.; Martínez, B. Model for Listeria monocytogenes inactivation by high hydrostatic pressure processing in Spanish chorizo sausage. Food Microbiol. 2018, 69, 18–24. [Google Scholar] [CrossRef]
- Bonilauri, P.; Grisenti, M.S.; Daminelli, P.; Merialdi, G.; Ramini, M.; Bardasi, L.; Taddei, R.; Cosciani-Cunico, E.; Dalzini, E.; Frustoli, M.A.; et al. Reduction of Salmonella spp. populations in Italian salami during production process and high pressure processing treatment: Validation of processes to export to the U.S. Meat Sci. 2019, 157, 107869. [Google Scholar] [CrossRef]
- Bonilauri, P.; Merialdi, G.; Ramini, M.; Bardasi, L.; Taddei, R.; Silvia, M.; Daminelli, P.; Cosciani-cunico, E.; Dalzini, E.; Angela, M.; et al. Modeling the behavior of Listeria innocua in Italian salami during the production and high-pressure validation of processes for exportation to the. Meat Sci. 2021, 172, 108315. [Google Scholar] [CrossRef] [PubMed]
- Marcos, B.; Aymerich, T.; Garriga, M.; Arnau, J. Active packaging containing nisin and high pressure processing as post-processing listericidal treatments for convenience fermented sausages. Food Control 2013, 30, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Cuevas, M.; Guevara, L.; Aznar, A.; Martínez, A.; Periago, P.M.; Fernández, P.S. Characterisation of the resistance and the growth variability of Listeria monocytogenes after high hydrostatic pressure treatments. Food Control 2013, 29, 409–415. [Google Scholar] [CrossRef]
- Balamurugan, S.; Gemmell, C.; Lau, A.T.Y.; Arvaj, L.; Strange, P.; Gao, A.; Barbut, S. High pressure processing during drying of fermented sausages can enhance safety and reduce time required to produce a dry fermented product. Food Control 2020, 113, 107224. [Google Scholar] [CrossRef]
- Liu, Y.; Betti, M.; Gänzle, M.G. High pressure inactivation of Escherichia coli, Campylobacter jejuni, and spoilage microbiota on poultry meat. J. Food Prot. 2012, 75, 497–503. [Google Scholar] [CrossRef]
- Rocchetti, G.; Falasconi, I.; Dallolio, G.; Lucini, L.; Rebecchi, A. Impact of hurdle technologies and low temperatures during ripening on the production of nitrate-free pork salami: A microbiological and metabolomic comparison. LWT 2021, 141, 110939. [Google Scholar] [CrossRef]
- Hospital, X.F.; Hierro, E.; Stringer, S.; Fernández, M. A study on the toxigenesis by Clostridium botulinum in nitrate and nitrite-reduced dry fermented sausages. Int. J. Food Microbiol. 2016, 218, 66–70. [Google Scholar] [CrossRef]
- ISO21807:2004 Microbiology of Food and Animal Feeding Stuffs—Determination of Water Activity; ISO: Geneva, Switzerland, 2004.
- ISO15213:2003 Microbilogy of Food and Feeding Stuffs-Horizontal Method for the Enumeration od Sulfite-Reducing Bacteria Growing under Anaerobic Conditions; ISO: Geneva, Switzerland, 2003.
- ISO6888-1:1999 Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus Aureus and other Species)—Part 1: Technique Using Baird-Parker Agar Medium; ISO: Geneva, Switzerland, 1999.
- ISO7932:2004 Microbiology of Food and Feeding Stuffs-Horizontal Method for the Enumeration of Presumptive Bacillus Cereus–Colony-count Technique at 30 Degrees C; ISO: Geneva, Switzerland, 2004.
- ISO11290-2:2017 Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes and of Listeria Spp.—Part 2: Enumeration Method; ISO: Geneva, Switzerland, 2017.
- ISO11290-1:2017 Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes and of Listeria Spp.—Part 1: Detection Method. International; ISO: Geneva, Switzerland, 2017.
- ISO6579-1:2017 Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella Spp; ISO: Geneva, Switzerland, 2007.
- ISO18787:2017 Foodstuffs- Determination of Water Activity; ISO: Geneva, Switzerland, 2017.
- Cardinali, F.; Milanović, V.; Osimani, A.; Aquilanti, L.; Taccari, M.; Garofalo, C.; Polverigiani, S.; Clementi, F.; Franciosi, E.; Tuohy, K.; et al. Microbial dynamics of model Fabriano-like fermented sausages as affected by starter cultures, nitrates and nitrites. Int. J. Food Microbiol. 2018, 278, 61–72. [Google Scholar] [CrossRef]
- Mataragas, M.; Bellio, A.; Rovetto, F.; Astegiano, S.; Greci, C.; Hertel, C.; Decastelli, L.; Cocolin, L. Quantification of persistence of the food-borne pathogens Listeria monocytogenes and Salmonella enterica during manufacture of Italian fermented sausages. Food Control 2015, 47, 552–559. [Google Scholar] [CrossRef]
- Higuero, N.; Moreno, I.; Lavado, G.; Vidal-Aragón, M.C.; Cava, R. Reduction of nitrate and nitrite in Iberian dry cured loins and its effects during drying process. Meat Sci. 2020, 163, 108062. [Google Scholar] [CrossRef]
- Pini, F.; Aquilani, C.; Giovannetti, L.; Viti, C.; Pugliese, C. Characterization of the microbial community composition in Italian Cinta Senese sausages dry-fermented with natural extracts as alternatives to sodium nitrite. Food Microbiol. 2020, 89, 103417. [Google Scholar] [CrossRef]
- Eyiler, E.; Oztan, A. Production of frankfurters with tomato powder as a natural additive. LWT Food Sci. Technol. 2011, 44, 307–311. [Google Scholar] [CrossRef]
- Hayes, J.E.; Canonico, I.; Allen, P. Effects of organic tomato pulp powder and nitrite level on the physicochemical, textural and sensory properties of pork luncheon roll. MESC 2013, 95, 755–762. [Google Scholar] [CrossRef]
- Patarata, L.; Martins, S.; Silva, J.A.; Fraqueza, M.J. Red wine and garlic as a possible alternative to minimize the use of nitrite for controlling Clostridium sporogenes and Salmonella in a cured sausage: Safety and sensory implications. Foods 2020, 9, 206. [Google Scholar] [CrossRef] [Green Version]
- Marcos, B.; Aymerich, T.; Garriga, M. Evaluation of High Pressure Processing as an Additional Hurdle to Control Listeria monocytogenes and Salmonella enterica in Low-Acid Fermented Sausages. Food Microbiol. Saf. 2005, 70, 339–344. [Google Scholar] [CrossRef]
- Martin, I.; Rodriguez, A.; Sanchez-Montero, L.; Padilla, P.; Cordoba, J.J. Effect of the Dry-Cured Fermented Sausage “Salchichón ” Processing with a selected Lactobacillus sakei in Listeria monocytogenes and Microbial Population. Foods 2021, 10, 856. [Google Scholar] [CrossRef]
- EC 2073/2005 EC Commission Regulation No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, 338, 1–26.
- Hayman, M.M.; Baxter, I.; O’Riordan, P.J.; Stewart, C.M. Effects of high-pressure processing on the safety, quality, and shelf life of ready-to-eat meats. J. Food Prot. 2004, 67, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-fandos, E.; De Castro, M.V.; Martinez-laorden, A.; Perez-arnedo, I. Behavior of Listeria monocytogenes and Other Microorganisms in Sliced Riojano Chorizo (Spanish Dry-Cured Sausage) during Storage under Modified Atmospheres. Microorganisms 2021, 9, 1384. [Google Scholar] [CrossRef]
- Van Reckem, E.; Geeraerts, W.; Charmpi, C.; Van Der Veken, D. Exploring the Link Between the Geographical Origin of European Fermented Foods and the Diversity of Their Bacterial Communities: The Case of Fermented Meats. Front. Microbiol. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Oliveira, M.; Ferreira, V.; Magalhães, R.; Teixeira, P. Biocontrol strategies for Mediterranean-style fermented sausages. Food Res. Int. 2018, 103, 438–449. [Google Scholar] [CrossRef]
- Peña-Meléndez, M.; Perry, J.J.; Yousef, A. Changes in thermal resistance of three Salmonella serovars in response to osmotic shock and adaptation at water activities reduced by different humectants. J. Food Prot. 2014, 77, 914–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farakos, S.M.S.; Frank, J.F.; Schaffner, D.W. International Journal of Food Microbiology Modeling the influence of temperature, water activity and water mobility on the persistence of Salmonella in low-moisture foods. Int. J. Food Microbiol. 2013, 166, 280–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finn, S.; Condell, O.; Mcclure, P.; Amézquita, A.; Fanning, S. Mechanisms of survival, responses, and sources of Salmonella in low-moisture environments. Front. Microbiol. 2013, 4, 331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balamurugan, S.; Ahmed, R.; Chibeu, A.; Gao, A.; Koutchma, T.; Strange, P. International Journal of Food Microbiology Effect of salt types and concentrations on the high-pressure inactivation of Listeria monocytogenes in ground chicken. Int. J. Food Microbiol. 2016, 218, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Bover-Cid, S.; Belletti, N.; Aymerich, T.; Garriga, M. Modelling the impact of water activity and fat content of dry-cured ham on the reduction of Salmonella enterica by high pressure processing. MESC 2017, 123, 120–125. [Google Scholar] [CrossRef]
- Hayman, M.M.; Kouassi, G.K.; Anantheswaran, R.C.; Floros, J.D.; Knabel, S.J. Effect of water activity on inactivation of Listeria monocytogenes and lactate dehydrogenase during high pressure processing. Int. J. Food Microbiol. 2008, 124, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Koseki, S.; Yamamoto, K. Water activity of bacterial suspension media unable to account for the baroprotective effect of solute concentration on the inactivation of Listeria monocytogenes by high hydrostatic pressure. Int. J. Food Microbiol. 2007, 115, 43–47. [Google Scholar] [CrossRef]


| Identification Number | Specie | Source of Isolation |
|---|---|---|
| IZSLER 111373/1 | L. innocua | Environmental swab of sausage factory |
| IZSLER 111373/2 | L. innocua | Environmental swab of sausage factory |
| IZSLER 257529/1 | L. innocua | Fresh sausage |
| IZSLER 257529/2 | L. innocua | Pork meat |
| ATCC 33090 | L. innocua | Cow brain |
| IZSLER 118174/1 | S. enterica subsp. enterica serovar Typhimurium | Fresh sausage |
| IZSLER 106463/1 | S. enterica serovar Derby | Pork meat |
| ATCC 14028 | S. enterica subsp. enterica serovar Typhimurium | Animal tissues |
| Time (Days) | L. innocua | Salmonella | Lactobacilli | CNS | pH | aw |
| 0 | 7.12 ± 0.01 e | 6.87 ± 0.05 d | 6.96 ± 0.09 a | 6.62 ± 0.10 c | 5.22 ± 0.01 a | 0.971 ± 0.001 c |
| 6 | 6.41 ± 0.03 d | 7.03 ± 0.08 d | 8.12 ± 0.24 c | 6.93 ± 0.18 c | 5.25 ± 0.02 a | 0.964 ± 0.003 c |
| 35 (before HPP) | 5.54 ± 0.12 c | 6.02 ± 0.13 c | 8.00 ± 0.29 c | 7.27 ± 0.35 c | 5.25 ± 0.03 a | 0.896 ± 0.007 b |
| 35 (after HPP) | 2.38 ± 0.42 a | 3.04 ± 0.07 a | 6.73 ± 0.42 a | 3.73 ± 0.54 a | ND | ND |
| 65 (Non-treated) | 4.78 ± 0.17 b | 3.94 ± 0.26 b | 7.46 ± 0.24 b | 5.40 ± 0.71 b | 5.34 ± 0.03 b | 0.882 ± 0.008 a |
| Δ [log (N2/N1)] | L. innocua | Salmonella | Lactobacilli | CNS | ||
| Δ Ripening | −1.58 | −0.85 | 1.05 | 0.65 | ||
| Δ HPP | −3.16 | −2.98 | −1.27 | −3.54 | ||
| Δ Ripening + Δ HPP | −4.74 | −3.83 | −0.23 | −2.89 | ||
| Δ Storage | −0.76 | −2.07 | −0.54 | −1.87 | ||
| Δ Ripening + Δ Storage | −2.34 | −2.92 | 0.51 | −1.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez, C.M.; Dallolio, G.; Bonilauri, P.; Rebecchi, A. Strategies for Nitrite Replacement in Fermented Sausages and Effect of High Pressure Processing against Salmonella spp. and Listeria innocua. Foods 2021, 10, 2617. https://doi.org/10.3390/foods10112617
Lopez CM, Dallolio G, Bonilauri P, Rebecchi A. Strategies for Nitrite Replacement in Fermented Sausages and Effect of High Pressure Processing against Salmonella spp. and Listeria innocua. Foods. 2021; 10(11):2617. https://doi.org/10.3390/foods10112617
Chicago/Turabian StyleLopez, Constanza Maria, Giuliano Dallolio, Paolo Bonilauri, and Annalisa Rebecchi. 2021. "Strategies for Nitrite Replacement in Fermented Sausages and Effect of High Pressure Processing against Salmonella spp. and Listeria innocua" Foods 10, no. 11: 2617. https://doi.org/10.3390/foods10112617
APA StyleLopez, C. M., Dallolio, G., Bonilauri, P., & Rebecchi, A. (2021). Strategies for Nitrite Replacement in Fermented Sausages and Effect of High Pressure Processing against Salmonella spp. and Listeria innocua. Foods, 10(11), 2617. https://doi.org/10.3390/foods10112617