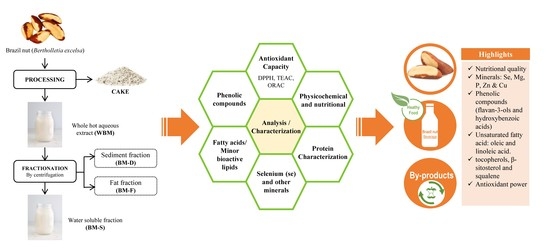
Composition of Brazil Nut (Bertholletia excels HBK), Its Beverage and By-Products: A Healthy Food and Potential Source of Ingredients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Solvents, Reagents, and Standards
2.3. Production of Brazil Nut Beverage
2.4. Methods of Analysis
2.4.1. Physicochemical Analysis
2.4.2. Phenolic Compounds
Extraction
Total phenolics Content (TPC)
Individual Phenolic Compounds
2.4.3. Lipids and Other Minor Lipidic Compounds
Extraction
Fatty Acids
Minor Lipid Compounds: Free Fatty Acids, Monoglycerides, Tocopherols, Phytosterol, and Squalene
2.4.4. Proteins
SDS-PAGE Analysis
Mass Spectrometry Analysis of Peptides
2.4.5. Minerals
Selenium
Other Minerals
2.4.6. Antioxidant Capacity
DPPH Assay
TEAC Assay
ORAC Assay
2.4.7. Statistical Analysis
3. Results and Discussion
3.1. Production of Brazil Nut Beverage
3.2. Physicochemical Properties
3.3. Phenolic Compounds
3.3.1. Total Phenolic Content (TPC)
3.3.2. Identification of Individual Soluble Phenolic Compounds
| Peak 1 | Rt (min) | Assigned Identity | UV λmax (nm) | [M-H]− m/z | MS/MS m/z | References |
|---|---|---|---|---|---|---|
| 1 | 2.9 | Citric acid 2 | 209 | 191 | 111, 173 | [12,34] |
| 2 | 3.1 | Pyruvic acid 2 | 227.8 (259.7) | 87.06 | 59.01 | [36] |
| 3 | 3.8 | Ascorbic acid 2 | 289 | 175.03 | 147.2, 87.00, 69.03 | [34] |
| 4 | 4.8 | Succinic acid 2 | 228 | 117.02 | 72.91 | [35] |
| 5 | 6.4 | Gallic acid | 274 | 169.01 | 125.02, 107.01, 97.03, 79.02, 69.03, 51.02, 41.04 | [37] |
| 6 | 6.9 | Gallic acid derivative | 272 | 187 | 125.02, 169.01 | [12] |
| 7 | 21.7 | Protocatechuic acid derivative | 280 | − | 153.04, 109.03, 124.03 | [12] |
| 8 | 22.3 | Catechin | 230, 280 | 289.1 | 136.8, 150.7, 160.8 | [12,38] |
| 9 | 24.0 | Catechin derivative | 282 | − | 289.1 | [12] |
| 10 | 28.4 | 4-hydroxybenzoic acid | 252 | 137.03 | 106.64, 93.03 | [38] |
| 11 | 30.2 | Vanillic acid | 259, 292 | 167.03 | 152.01, 108.02 | [12,39] |
| 12 | 30.9 | Epicatechin | 279 | 289.1 | 109.01, 121.01, 123.03, 125.01, 137.00 | [38] |
| 13 | 32.4 | Vanillin | 274, 309 | 151.05 | 137.05, 123.05, 109.0, 81.0 | [40] |
| 14 | 33.4 | Catechin gallate | 231.8, 280.1, (324.8) | 441.03 | 109.01, 125.00, 168.98, 289.03 | [41,42] |
| 15 | 33.8 | Epicatechin gallate | 232.6, 280 (324.8) | 441.19 | 109.08, 125.08,137.08, 151.10, 203.14, 245.16, 289.16 | [38] |
| 16 | 34.6 | Epigallocatechin 3-O gallate | 232; 280 (312) | 457.3 | 305.6, 169.1, 125.02 | [43] |
| 17 | 35.4 | p-Coumaric acid | 227, 310 | 164.05 | 119.05, 91.05 | [39] |
| 18 | 36.4 | Ferulic acid | (292), 323 | 193.1 | 177.1, 161, 133.1 | [39] |
| 19 | 37.0 | Taxifolin (dihydroquercetin) | 231.9, (282.8) 309.2 | 303.05 | 285.05, 179.00, 125.03 | [12] |
| 20 | 37.9 | Ellagic acid derivative | 252, 360 | 447 | 301, 257, 229 | [12] |
| 21 | 38.9 | Quercetin | 232, 323 | 301 | 179, 151 | [12,44] |
| 22 | 39.7 | Ellagic acid | 254, 368 | 301 | 285, 283, 257, 229, 184.92, 134.92 | [12] |
| 23 | 40.0 | Myricetin-3-O-rhamnoside | 253, 370 | 463 | 317 | [12,45] |
| 24 | 42.1 | Vanillic acid derivative | 259, 294 | 329 | 167 | [12] |
3.3.3. Individual Phenolic Compounds Content in BN and Derivates
3.4. Lipids and Other Lipophilic Components
3.4.1. Fatty Acid Profile
3.4.2. Free Fatty Acids and Monoglycerides
3.4.3. Phytosterols, Tocopherols, and Squalene
3.5. Characterization of Proteins in Brazil Nut and Derivatives
3.6. Selenium and Other Minerals
3.6.1. Selenium Content
3.6.2. Other Minerals Content
| Minerals (mg/100 g) | Raw Material | Beverages | By-Products | DRV 3 (mg/Day) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BN 1 | WBM 1 | BM-S 1 | BM-D 2 | BM-F 2 | Cake 2 | ||||||||
| Mg | 468.85 | ±25 a | 35.89 | ±1.1 b | 11.06 | ±0.67 b | 2301.5 | ±62 c | 19.27 | ±1.06 b | 513.03 | ±8.8 a | 325 |
| K | 576.21 | ±40 a | 77.70 | ±0.7 b | 55.13 | ±1.25 b | 1949.6 | ±108 d | 38.92 | ±1.89 b | 421.15 | ±44 c | 3500 |
| Ca | 186.83 | ±2.6 a | 13.96 | ±2.3 a | 2.36 | ±0.27 a | 1196 | ±227 b | 7.79 | ±1.18 a | 221.62 | ±1.6 a | 1000 |
| P | 686.05 | ±13 a | 60.59 | ±2.3 b | 11.38 | ±1.16 b | 4605 | ±151 c | 23.17 | ±4.38 b | 725.11 | ±11.6 a | 550 |
| Zn | 4.72 | ±0.1 a | 0.43 | ±0.01 b | 0.02 | ±0.00 d | 36.4 | ±0.27 e | 0.59 | ±0.08 b | 5.71 | ±0.1 c | 11.5 |
| Cu | 1.83 | ±0.1 a | 0.21 | ±0.0 b | 0.16 | ±0.00 b | 3.86 | ±0.03 d | 0.16 | ±0.02 b | 1.48 | ±0.0 c | 1.5 |
| Fe | 3.12 | ±0.1 a | 0.22 | ±0.0 bd | 0.01 | ±0.00 d | 19.04 | ±0.22 e | 0.31 | ±0.05 b | 3.40 | ±0.01 c | 11.0 |
| Mn | 0.75 | ±0.1 a | 0.07 | ±0.0 b | 0.01 | ±0.00 b | 6.66 | ±0.14 e | <1.1 b | 1.06 | ±0.02 c | 3 | |
| Se (μg/100g) | 3628 | ±51 a | 15 | ±0.2 d | 8.3 | ±0.14 d | 537 | ±6 b | 19 | ±0.3 d | 229 | ±3 c | 70 (µg/day) |
3.7. Antioxidant Capacity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kainer, K.A.; Wadt, L.H.O.; Staudhammer, C.L. The evolving role of Bertholletia excelsa in Amazonia: Contributing to local livelihoods and forest conservation. Desenvolv. Meio Ambient. 2018, 48, 477–497. [Google Scholar] [CrossRef]
- Eslami, O.; Shidfar, F.; Dehnad, A. Inverse association of long-term nut consumption with weight gain and risk of overweight/obesity: A systematic review. Nutr. Res. 2019, 68, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.R.; Duarte, G.B.S.; Reis, B.Z.; Cozzolino, S.M.F. Brazil nuts: Nutritional composition, health benefits and safety aspects. Food Res. Int. 2017, 100, 9–18. [Google Scholar] [CrossRef]
- Chalupa-Krebzdak, S.; Long, C.J.; Bohrer, B.M. Nutrient density and nutritional value of milk and plant-based milk alternatives. Int. Dairy J. 2018, 87, 84–92. [Google Scholar] [CrossRef]
- Aydar, E.F.; Tutuncu, S.; Ozcelik, B. Plant-based milk substitutes: Bioactive compounds, conventional and novel processes, bioavailability studies, and health effects. J. Funct. Foods 2020, 70, 103975. [Google Scholar] [CrossRef]
- Felberg, I.; Antoniassi, R.; Deliza, R.; de Freitas, S.C.; Modesta, R.C. Della Bebida de soja e castanha do Brasil: Processamento, composição, avaliação sensorial e de cor. Cienc. Tecnol. Aliment. 2009, 29, 609–617. [Google Scholar] [CrossRef] [Green Version]
- Cardarelli, H.R.; Joaquim, A. Conservation of Brazil Nut extract. Sci. Agric. 2000, 57, 617–622. [Google Scholar] [CrossRef] [Green Version]
- Kluczkovski, A.; Lima, N.; Oliveira, M.K. Brazil nut powdered milk properties. J. Food Process. Preserv. 2017, 41, 1–6. [Google Scholar] [CrossRef]
- Sartori, A.G.D.O.; Machado, M.C.; Bastos, D.H.M.; de Alencar, S.M.; Regitano-d’Arce, M.A.B. Water-extracted Brazil nut co-products: Nutritional value and estimation of nutrient losses during processing. J. Food Meas. Charact. 2020, 14, 1919–1925. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Method of Analysis, 18th ed.; AOAC International: Washington, DC, USA, 2005; ISBN 9780935584424. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple technique to rule out occlusion of right coronary artery after aortic valve surgery. J. Biol. Chem. 1956, 226, 497–509. [Google Scholar] [CrossRef]
- John, J.A.; Shahidi, F. Phenolic compounds and antioxidant activity of Brazil nut (Bertholletia excelsa). J. Funct. Foods 2010, 2, 196–209. [Google Scholar] [CrossRef]
- Cano, M.P.; Gómez-Maqueo, A.; García-Cayuela, T.; Welti-Chanes, J. Characterization of carotenoid profile of Spanish Sanguinos and Verdal prickly pear (Opuntia ficus-indica, spp.) tissues. Food Chem. 2017, 237, 612–622. [Google Scholar] [CrossRef] [PubMed]
- García-Cayuela, T.; Gómez-Maqueo, A.; Guajardo-Flores, D.; Welti-Chanes, J.; Cano, M.P. Characterization and quantification of individual betalain and phenolic compounds in Mexican and Spanish prickly pear (Opuntia ficus-indica L. Mill) tissues: A comparative study. J. Food Compos. Anal. 2019, 76, 1–13. [Google Scholar] [CrossRef]
- Herrera, T.; Navarro del Hierro, J.; Fornari, T.; Reglero, G.; Martin, D. Acid hydrolysis of saponin-rich extracts of quinoa, lentil, fenugreek and soybean to yield sapogenin-rich extracts and other bioactive compounds. J. Sci. Food Agric. 2019, 99, 3157–3167. [Google Scholar] [CrossRef] [Green Version]
- Sanchón, J.; Fernández-Tomé, S.; Miralles, B.; Hernández-Ledesma, B.; Tomé, D.; Gaudichon, C.; Recio, I. Protein degradation and peptide release from milk proteins in human jejunum. Comparison with in vitro gastrointestinal simulation. Food Chem. 2018, 239, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Miralles, B.; Sanchón, J.; Sánchez-Rivera, L.; Martínez-Maqueda, D.; Le Gouar, Y.; Dupont, D.; Amigo, L.; Recio, I. Digestion of micellar casein in duodenum cannulated pigs. Correlation between in vitro simulated gastric digestion and in vivo data. Food Chem. 2021, 343, 128424. [Google Scholar] [CrossRef] [PubMed]
- López-Bellido, F.J.; Sanchez, V.; Rivas, I.; López-Bellido, R.J.; López-Bellido, L. Wheat grain selenium content as affected by year and tillage system in a rainfed Mediterranean Vertisol. F. Crop. Res. 2019, 233, 41–48. [Google Scholar] [CrossRef]
- Nielsen, S.S. Food Analysis, 5th ed.; Springer Nature: West Lafayette, IN, USA, 2017; ISBN 9783319457741. [Google Scholar]
- Abe, L.T.; Lajolo, F.M.; Genovese, M.I. Comparison of phenol content and antioxidant capacity of nuts. Ciênc. Tecnol. Aliment. 2010, 30, 254–259. [Google Scholar] [CrossRef] [Green Version]
- Koroleva, O.; Torkova, A.; Nikolaev, I.; Khrameeva, E.; Fedorova, T.; Tsentalovich, M.; Amarowicz, R. Evaluation of the antiradical properties of phenolic acids. Int. J. Mol. Sci. 2014, 15, 16351–16380. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Maqueo, A.; García-Cayuela, T.; Welti-Chanes, J.; Cano, M.P. Enhancement of anti-inflammatory and antioxidant activities of prickly pear fruits by high hydrostatic pressure: A chemical and microstructural approach. Innov. Food Sci. Emerg. Technol. 2019, 54, 132–142. [Google Scholar] [CrossRef]
- Silva, A.R.A.; Silva, M.M.N.; Ribeiro, B.D. Health issues and technological aspects of plant-based alternative milk. Food Res. Int. 2020, 131, 108972. [Google Scholar] [CrossRef]
- McClements, D.J.; Newman, E.; McClements, I.F. Plant-based Milks: A Review of the Science Underpinning Their Design, Fabrication, and Performance. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2047–2067. [Google Scholar] [CrossRef] [Green Version]
- Shimoyamada, M.; Tsushima, N.; Tsuzuki, K.; Asao, H.; Yamauchi, R. Effect of Heat Treatment on Dispersion Stability of Soymilk and Heat Denaturation of Soymilk Protein. Food Sci. Technol. Res. 2008, 14, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Devnani, B.; Ong, L.; Kentish, S.; Gras, S. Heat induced denaturation, aggregation and gelation of almond proteins in skim and full fat almond milk. Food Chem. 2020, 325, 126901. [Google Scholar] [CrossRef]
- Santos, O.V.; Corrêa, N.C.F.; Carvalho, R.N.; Costa, C.E.F.; França, L.F.F.; Lannes, S.C.S. Comparative parameters of the nutritional contribution and functional claims of Brazil nut kernels, oil and defatted cake. Food Res. Int. 2013, 51, 841–847. [Google Scholar] [CrossRef]
- Silva, J.G.S.; Rebellato, A.P.; Caramês, E.T. dos S.; Greiner, R.; Pallone, J.A.L. In vitro digestion effect on mineral bioaccessibility and antioxidant bioactive compounds of plant-based beverages. Food Res. Int. 2020, 130, 108993. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Zeng, X.A.; Ahmad, N.; Ahmed, Z.; Rehman, A.; Aadil, R.M.; Roobab, U.; Siddique, R.; Rahaman, A. Effect of pulsed electric field and thermal treatments on the bioactive compounds, enzymes, microbial, and physical stability of almond milk during storage. J. Food Process. Preserv. 2020, 44, 1–14. [Google Scholar] [CrossRef]
- Gomes, S.; Torres, A.G. Optimized extraction of polyphenolic antioxidant compounds from Brazil nut (Bertholletia excelsa) cake and evaluation of the polyphenol profile by HPLC. J. Sci. Food Agric. 2015, 96, 2805–2814. [Google Scholar] [CrossRef]
- Chen, P.C.; Lin, C.; Chen, M.H.; Chiang, P.Y. The micronization process for improving the dietary value of okara (soybean residue) by planetary ball milling. LWT 2020, 132, 109848. [Google Scholar] [CrossRef]
- Lazarin, R.A.; Falcão, H.G.; Ida, E.I.; Berteli, M.N.; Kurozawa, L.E. Rotating-Pulsed Fluidized Bed Drying of Okara: Evaluation of Process Kinetic and Nutritive Properties of Dried Product. Food Bioprocess Technol. 2020, 13, 1611–1620. [Google Scholar] [CrossRef]
- Miraliakbari, H.; Shahidi, F. Antioxidant activity of minor components of tree nut oils. Food Chem. 2008, 111, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Spínola, V.; Pinto, J.; Castilho, P.C. Identification and quantification of phenolic compounds of selected fruits from Madeira Island by HPLC-DAD-ESI-MSn and screening for their antioxidant activity. Food Chem. 2015, 173, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Mata, A.; Ferreira, J.P.; Semedo, C.; Serra, T.; Duarte, C.M.M.; Bronze, M.R. Contribution to the characterization of Opuntia spp. juices by LC-DAD-ESI-MS/MS. Food Chem. 2016, 210, 558–565. [Google Scholar] [CrossRef]
- Bylund, D.; Norström, S.H.; Essén, S.A.; Lundström, U.S. Analysis of low molecular mass organic acids in natural waters by ion exclusion chromatography tandem mass spectrometry. J. Chromatogr. A 2007, 1176, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Arráez-Román, D.; Fu, S.; Sawalha, S.M.S.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC/CE-ESI-TOF-MS methods for the characterization of polyphenols in almond-skin extracts. Electrophoresis 2010, 31, 2289–2296. [Google Scholar] [CrossRef]
- Milbury, P.E.; Chen, C.Y.; Dolnikowski, G.G.; Blumberg, J.B. Determination of flavonoids and phenolics and their distribution in almonds. J. Agric. Food Chem. 2006, 54, 5027–5033. [Google Scholar] [CrossRef]
- Pycia, K.; Kapusta, I.; Jaworska, G.; Jankowska, A. Antioxidant properties, profile of polyphenolic compounds and tocopherol content in various walnut (Juglans regia L.) varieties. Eur. Food Res. Technol. 2019, 245, 607–616. [Google Scholar] [CrossRef]
- Bueno-Herrera, M.; Pérez-Magariño, S. Validation of an extraction method for the quantification of soluble free and insoluble bound phenolic compounds in wheat by HPLC-DAD. J. Cereal Sci. 2020, 93, 102984. [Google Scholar] [CrossRef]
- Jaiswal, R.; Jayasinghe, L.; Kuhnert, N. Identification and characterization of proanthocyanidins of 16 members of the Rhododendron genus (Ericaceae) by tandem LC-MS. J. Mass Spectrom. 2012, 47, 502–515. [Google Scholar] [CrossRef]
- Ojeda-Amador, R.M.; Salvador, M.D.; Fregapane, G.; Gómez-Alonso, S. Comprehensive Study of the Phenolic Compound Profile and Antioxidant Activity of Eight Pistachio Cultivars and Their Residual Cakes and Virgin Oils. J. Agric. Food Chem. 2019, 67, 3583–3594. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, P.; Lin, L.; Harnly, J.M.; Yu, L.; Li, Z. Tentative identification, quantitation, and principal component analysis of green pu-erh, green, and white teas using UPLC/DAD/MS. Food Chem. 2011, 126, 1269–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regueiro, J.; Sánchez-gonzález, C.; Vallverdú-queralt, A.; Simal-gándara, J.; Lamuela-raventós, R.; Izquierdo-pulido, M. Comprehensive identification of walnut polyphenols by liquid chromatography coupled to linear ion trap—Orbitrap mass spectrometry. Food Chem. 2014, 152, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Slatnar, A.; Mikulic-petkovsek, M.; Stampar, F.; Veberic, R.; Solar, A. HPLC-MS n identi fi cation and quanti fi cation of phenolic compounds in hazelnut kernels, oil and bagasse pellets. Food Res. Int. 2014, 64, 783–789. [Google Scholar] [CrossRef]
- Bodoira, R.; Maestri, D. Phenolic Compounds from Nuts: Extraction, Chemical Profiles, and Bioactivity. J. Agric. Food Chem. 2020, 68, 927–942. [Google Scholar] [CrossRef]
- Bhagwat, S.; Haytowitz, D.B. USDA Database for the Flavonoid Content of Selected Foods. Release 3.2; USDA NAL: Maryland, MD, USA, 2015.
- Özcan, M.M.; Al Juhaimi, F.; Uslu, N. The effect of heat treatment on phenolic compounds and fatty acid composition of Brazilian nut and hazelnut. J. Food Sci. Technol. 2018, 55, 376–380. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K.C. Isoflavones, flavan-3-ols, phenolic acids, total phenolic profiles, and antioxidant capacities of soy milk as affected by ultrahigh-temperature and traditional processing methods. J. Agric. Food Chem. 2009, 57, 4706–4717. [Google Scholar] [CrossRef]
- Favari, C.; Mena, P.; Curti, C.; Del, D. Flavan-3-ols: Catechins and Proanthocyanidins. In Dietary Polyphenols: Metabolism and Health Effect; Tomás-Barberán, F.A., González-Sarrías, A., García-Villalba, R., Eds.; John Wiley & Sons, Inc.: Chennai, India, 2021; pp. 283–317. ISBN 9781119563716. [Google Scholar]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. J. Parenter. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef] [PubMed]
- Weston, M.; Kuchel, R.P.; Chandrawati, R. A Polydiacetylene-Based Colorimetric Sensor as an Active Use-By Date for Plant-Based Milk Alternatives. Macromol. Rapid Commun. 2020, 41, 2000172. [Google Scholar] [CrossRef]
- Santos, O.V.; Corrêa, N.C.F.; Soares, F.A.S.M.; Gioielli, L.A.; Costa, C.E.F.; Lannes, S.C.S. Chemical evaluation and thermal behavior of Brazil nut oil obtained by different extraction processes. Food Res. Int. 2012, 47, 253–258. [Google Scholar] [CrossRef]
- Union Europea. Regulation (EU) No 1308/2013. Establishing a Common Organisation of the Markets in Agricultural Products. Available online: https://eur-lex.europa.eu (accessed on 12 March 2020).
- Kundu, P.; Dhankhar, J.; Sharma, A. Development of Non Dairy Milk Alternative Using Soymilk and Almond Milk. Curr. Res. Nutr. Food Sci. 2018, 06, 203–210. [Google Scholar] [CrossRef]
- Shahidi, F.; Camargo, A.C. De Tocopherols and Tocotrienols in Common and Emerging Dietary Sources: Occurrence, Applications, and Health Benefits. Int. J. Mol. Sci. 2016, 17, 1745. [Google Scholar] [CrossRef] [PubMed]
- Derewiaka, D.; Szwed, E.; Wołosiak, R. Physicochemical properties and composition of lipid fraction of selected edible nuts. Pak. J. Bot. 2014, 46, 337–343. [Google Scholar]
- Lozano-Grande, M.A.; Gorinstein, S.; Espitia-Rangel, E.; Dávila-Ortiz, G.; Martínez-Ayala, A.L. Plant Sources, Extraction Methods, and Uses of Squalene. Int. J. Agron. 2018, 2018, 2–14. [Google Scholar] [CrossRef]
- Vasquez, W.V.; Hernández, D.M.; del Hierro, J.N.; Martin, D.; Cano, M.P.; Fornari, T. Supercritical carbon dioxide extraction of oil and minor lipid compounds of cake byproduct from Brazil nut (Bertholletia excelsa) beverage production. J. Supercrit. Fluids 2021, 171, 105188. [Google Scholar] [CrossRef]
- Sharma, G.M.; Mundoma, C.; Seavy, M.; Roux, K.H.; Sathe, S.K. Purification and Biochemical Characterization of Brazil nut (Bertholletia excelsa L.) Seed Storage Proteins. J. Agric. Food Chem. 2010, 58, 5714–5723. [Google Scholar] [CrossRef]
- Sathe, S.K.; Venkatachalam, M.; Sharma, G.M.; Kshirsagar, H.H.; Teuber, S.S.; Roux, K.H. Solubilization and Electrophoretic Characterization of Select Edible Nut Seed Proteins. J. Agric. Food Chem. 2009, 57, 7846–7856. [Google Scholar] [CrossRef] [PubMed]
- Ampe, C.; Van Damme, J.; de Castro, L.A.B.; Sampaio, M.J.A.M.; Van Montagu, M.; Vandekerckhove, J. The amino-acid sequence of the 2S sulphur-rich proteins from seeds of Brazil nut (Bertholletia excelsa H.B.K.). Eur. J. Biochem. 1986, 159, 597–601. [Google Scholar] [CrossRef]
- Lima, L.W.; Stonehouse, G.C.; Walters, C.; El Mehdawi, A.F.; Fakra, S.C.; Pilon-Smits, E.A.H. Selenium accumulation, speciation and localization in Brazil nuts (Bertholletia excelsa H.B.K.). Plants 2019, 8, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Departamento de Agricultura de los Estados Unidos (USDA) USDA National Nutrient Database for Standard Reference, Release 28. Available online: https://ndb.nal.usda.gov/ndb/ (accessed on 10 January 2020).
- Jayasinghe, S.B.; Caruso, J.A. Investigation of Se-containing proteins in Bertholletia excelsa H.B.K. (Brazil nuts) by ICPMS, MALDI-MS and LC-ESI-MS methods. Int. J. Mass Spectrom. 2011, 307, 16–27. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Dietary Reference Values for nutrients Summary report. EFSA Support. Publ. 2017, 14, 1–98. [Google Scholar] [CrossRef] [Green Version]
- Vanga, S.K.; Raghavan, V. How well do plant based alternatives fare nutritionally compared to cow’s milk? J. Food Sci. Technol. 2018, 55, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Stanojevic, S.P.; Barac, M.B.; Pesic, M.B.; Zilic, S.M.; Kresovic, M.M.; Vucelic-Radovic, B.V. Mineral elements, lipoxygenase activity, and antioxidant capacity of okara as a byproduct in hydrothermal processing of soy milk. J. Agric. Food Chem. 2014, 62, 9017–9023. [Google Scholar] [CrossRef] [PubMed]



| Characteristic | Raw Material | Beverages | By-Products | |||
|---|---|---|---|---|---|---|
| BN 1 | WBM *,1 | BM-S 1 | BM-D 2 | BM-F 2 | Cake 2 | |
| Protein | 16.03 | 1.43 | 0.80 | 56.88 | 0.65 | 16.9 |
| Lipid | 58.52 | 5.24 | 0.25 | 14.6 | 81.3 | 44.2 |
| Carbohydrate | 19.61 | 1.94 | 0.83 | 16.65 | 16.29 | 35.45 |
| Ashes | 3.35 | 0.33 | 0.10 | 11.87 | 1.76 | 3.5 |
| Energy (Kcal) | 669.2 | 60.6 | 8.8 | 425.5 | 799.5 | 607.2 |
| Total solids 1 | 97.51 | 8.93 | 1.98 | 31.41 | 49.21 | 36.17 |
| Acidity 3 | 0.18 | 0.03 | 0.03 | 0.54 | - | 0.1 |
| pH | 6.4 | 6.5 | 6.5 | 6.7 | - | 6.6 |
| Soluble solid (°Brix) | 14.5 | 2.6 | 3.0 | 4.8 | - | 6.8 |
| Samples | TPC | ||||
|---|---|---|---|---|---|
| Dry Weight | Fresh Weight | ||||
| Raw material | BN | 108.0 | ±3.9 b | 105.3 | ±3.8 a |
| Beverages | WBM * | 79.5 | ±1.5 b | 7.1 | ±0.1 c |
| BM-S | 314.0 | ±5.2 a | 6.2 | ±0.1 c | |
| By-products | BM-D | 83.4 | ±3.1 b | 28.7 | ±1.1 b |
| Cake | 49.2 | ±2.7 c | 17.8 | ±1.0 b | |
| Rt (Min) | Phenolic Compound | BN 1 | WBM *,1 | BM-S 1 | BM-D 2 | Cake 2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic Acids | |||||||||||
| 6.4 | Gallic acid | 6.3 | ±0.2 | 0.7 | ±0.0 | 0.5 | ±0.0 | 17.7 | ±0.8 | 6.4 | ±0.3 |
| 6.9 | Gallic acid derivative | 6.9 | ±0.3 | 0.6 | ±0.0 | 0.4 | ±0.0 | 17.7 | ±0.2 | 6.5 | ±0.3 |
| 21.7 | Protocatechuic acid derivative | 42.0 | ±1.9 | 3.0 | ±0.1 | 2.1 | ±0.1 | 83.2 | ±4.0 | 31.7 | ±1.4 |
| 28.4 | 4-hydroxybenzoic acid | 6.5 | ±0.3 | 0.5 | ±0.0 | 0.4 | ±0.0 | 3.7 | ±0.0 | 2.2 | ±0.1 |
| 30.2 | Vanillic acid | 18.0 | ±0.8 | 1.5 | ±0.0 | 1.4 | ±0.0 | 4.9 | ±0.1 | 3.3 | ±0.1 |
| 35.4 | p-Coumaric acid | 8.5 | ±0.3 | 0.9 | ±0.0 | 0.8 | ±0.0 | 4.9 | ±0.1 | 2.6 | ±0.1 |
| 36.4 | Ferulic acid | 4.6 | ±0.2 | 0.5 | ±0.0 | 0.5 | ±0.0 | 1.7 | ±0.1 | 1.0 | ±0.0 |
| 42.1 | Vanillic acid derivative | 1.6 | ±0.1 | 0.2 | ±0.0 | 0.2 | ±0.0 | 0.6 | ±0.0 | 0.5 | ±0.0 |
| 37.9 | Ellagic acid derivative | 45.4 | ±0.4 | 4.7 | ±0.1 | 3.3 | ±0.1 | 138.6 | ±3.8 | 49.1 | ±1.7 |
| 39.7 | Ellagic acid | 36.2 | ±1.4 | 3.5 | ±0.0 | 2.1 | ±0.0 | 134.1 | ±3.4 | 46.8 | ±2.5 |
| Phenolic aldehyde | |||||||||||
| 32.4 | Vanillin | 5.6 | ±0.2 | 0.5 | ±0.0 | 0.5 | ±0.0 | 1.3 | ±0.1 | 1.2 | ±0.0 |
| Flavonoids | |||||||||||
| 22.3 | Catechin | 175.4 | ±7.9 | 21.6 | ±0.5 | 20.2 | ±0.5 | 127.6 | ±3.0 | 79.7 | ±3.3 |
| 24.0 | Catechin derivative | 86.1 | ±2.6 | 9.2 | ±0.5 | 8.9 | ±0.5 | 25.7 | ±1.2 | 18.3 | ±0.9 |
| 30.9 | Epicatechin | 21.8 | ±0.7 | 1.5 | ±0.0 | 1.4 | ±0.0 | 16.2 | ±0.4 | 8.0 | ±0.1 |
| 33.4 | Catechin-gallate | 71.0 | ±1.7 | 8.5 | ±0.1 | 8.2 | ±0.1 | 24.8 | ±0.6 | 16.7 | ±0.3 |
| 33.8 | Epicatechin-gallate | 14.3 | ±0.4 | 1.4 | ±0.0 | 1.3 | ±0.0 | 13.6 | ±0.2 | 6.3 | ±0.2 |
| 34.6 | Epigallocatechin 3-O-gallate | 7.4 | ±0.3 | 0.3 | ±0.0 | 0.2 | ±0.0 | 11.0 | ±0.3 | 4.3 | ±0.1 |
| 37.0 | Taxolin (dihydroquercetin) | -- | -- | -- | -- | -- | |||||
| 38.9 | Quercetin | 6.7 | ±0.2 | 0.9 | ±0.0 | 0.9 | ±0.0 | 0.2 | ±0.0 | 1.0 | ±0.0 |
| 40.0 | Myricetin-3-O-rhamnoside | 10.6 | ±0.5 | 0.2 | ±0.0 | 0.2 | ±0.0 | 6.8 | ±0.4 | 3.6 | ±0.0 |
| Total phenolic acids | 176.1 | ±5.8 | 16.0 | ±0.4 | 11.8 | ±0.3 | 407.1 | ±12.5 | 150.3 | ±6.5 | |
| Phenolic aldehyde | 5.6 | ±0.2 | 0.5 | ±0.0 | 0.5 | ±0.0 | 1.3 | ±0.1 | 1.2 | ±0.0 | |
| Total flavonoids | 393.4 | ±14.4 | 43.7 | ±1.2 | 41.1 | ±1.1 | 225.9 | ±6.1 | 138.0 | ±5.0 | |
| Total phenolic compounds | 575.0 | ±20.4 | 60.2 | ±1.6 | 53.3 | ±1.4 | 634.3 | ±18.6 | 289.4 | ±11.5 | |
| Fatty Acids (FA) % | BN | WBM Fractions | Cake | ||
|---|---|---|---|---|---|
| BM-S | BM-D | BM-F | |||
| Palmitic (C16:0) | 15.95 ± 1.06 a | 15.35 ± 0.15 a | 15.36 ± 0.05 a | 15.30 ± 0.05 a | 15.17 ± 0.11 a |
| Palmitoleic (C16:1) | 0.24 ± 0.01 b | 0.20 ± 0.02 b | 0.21 ± 0.02 b | 0.22 ± 0.02 b | 0.22 ± 0.01 b |
| Stearic (C18:0) | 10.32 ± 2.59 c | 9.84 ± 0.24 c | 10.09 ± 0.15 c | 9.57 ± 0.03 c | 9.52 ± 0.17 c |
| Oleic (C18:1) | 36.40 ± 5.68 d | 36.66 ± 1.23 d | 36.59 ± 0.34 d | 35.85 ± 0.18 d | 35.33 ± 0.45 d |
| Linoleic (C18:2) | 36.99 ± 7.22 e | 37.86 ± 1.30 e | 37.64 ± 0.47 e | 38.97 ± 0.10 e | 39.64 ± 0.52 e |
| Arachidic (C20:0) | 0.10 ± 0.06 f | 0.09 ± 0.01 f | 0.11 ± 0.03 f | 0.09 ± 0.01 f | 0.11 ± 0.02 f |
| Total Saturated (SFA) | 26.37 ± 3.71 g | 25.28 ± 0.39 g | 25.56 ± 0.23 g | 24.97 ± 0.09 g | 24.81 ± 0.30 g |
| Total Unsaturated (UFA) | 73.63 ±12.90 h | 74.72 ± 2.55 h | 74.44 ± 0.82 h | 75.03 ± 0.30 h | 75.19 ± 0.98 h |
| Polyunsaturated (PUFA) | 36.99 ± 7.22 i | 37.86 ± 1.30 i | 37.64 ± 0.47 i | 38.97 ± 0.10 i | 39.64 ± 0.52 i |
| Monounsaturated (MUFA) | 36.64 ± 5.69 j | 36.86 ± 1.25 j | 36.80 ± 0.36 j | 36.07 ± 0.19 j | 35.55 ± 0.46 j |
| Compound | Raw Material | Beverages | Byproducts | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BN 1 | WBM 1 | BM-S 1 | BM-D 2 | BM-F 2 | Cake 2 | ||||||||
| FFA | (mg/100 g) | 346 | ±61 a | 51.3 | ±12 ab | 3.11 | ±0.7 b | 181.4 | ±41 ab | 794 | ±172 c | 80.5 | ±4.1 ab |
| (g/100 g oil) | 0.59 | ±0.10 abc | 1.0 | ±0.2 abc | 1.24 | ±0.3 bc | 1.24 | ±0.28 b | 0.98 | ±0.2 abc | 0.18 | ±0.01 a | |
| Monoglycerides | (mg/100 g) | 110.8 | ±32 a | 113.5 | ±34 a | 5.66 | ±0.8 a | 304.3 | ±65 a | 1789 | ±58 b | 66.3 | ±33 a |
| (g/100 g oil) | 0.19 | ±0.05 a | 2.20 | ±0.6 b | 2.25 | ±0.3 b | 2.08 | ±0.45 b | 2.20 | ±0.6 b | 0.15 | ±0.07 a | |
| Total tocopherol | (mg/100 g) | 18.9 | ±6 a | 3.1 | ±0.3 c | 0.22 | ±0.04 bc | 7.2 | ±1.3 abc | 47.8 | ±5 d | 25.5 | ±0.8 ae |
| (mg/100 g oil) | 32.3 | ±11 a | 59.9 | ±6.1 b | 88.1 | ±17 b | 49.3 | ±9 ab | 58.77 | ±6 ab | 57.6 | ±1.8 ab | |
| γ-Tocopherol | (mg/100 g) | 16.5 | ±4.6 a | 2.4 | ±0.08 b | 0.18 | ±0.04 b | 5.76 | ±1.1 b | 37.4 | ±1 c | 19.9 | ±1.9 a |
| (mg/100 g oil) | 28.3 | ±7 ac | 47.1 | ±1.4 abc | 73.5 | ±14 b | 39.5 | ±7 c | 45.98 | ±1 abc | 45.1 | ±4.3 abc | |
| α-Tocopherol | (mg/100 g) | 2.4 | ±2 a | 0.7 | ±0.26 a | 0.04 | ±0.01 a | 1.4 | ±0.2 a | 10.4 | ±4 b | 5.5 | ±1.1 ab |
| (mg/100 g oil) | 4.02 | ±1.3 a | 12.8 | ±4.8 a | 14.61 | ±2.9 a | 9.78 | ±1.5 a | 12.79 | ±2.5 a | 12.5 | ±2.5 a | |
| β-Sitosterol | (mg/100 g) | 60.2 | ±15 a | 7.1 | ±0.9 b | 0.73 | ±0.23 b | 30.7 | ±6 ab | 105 | ±11 c | 62.1 | ±3 a |
| (mg/100 g oil) | 102.8 | ±26 b | 138.4 | ±17 ab | 289.5 | ±89 a | 210.1 | ±43 ab | 129 | ±13 ab | 140.4 | ±6 ab | |
| Squalene | (mg/100 g) | 217.5 | ±13 a | 13.2 | ±2.8 b | 0.69 | ±0.11 b | 40.1 | ±11 b | 207.6 | ±41 a | 123.6 | ±8 c |
| (mg/100 g oil) | 371.7 | ±23 a | 256.7 | ±50 a | 272.62 | ±43 a | 274.8 | ±74 a | 255.4 | ±50 a | 279.7 | ±18 a | |
| Band | Tryptic Peptide (Ion) | Protein Fragment | Sequence | Protein Name | Entry Number |
|---|---|---|---|---|---|
| B | 1145.44 | 194–202 | RSQKQRGER | 11S globulin | Q84ND2 (Bertholletia excelsa) |
| B | 1202.43 | 338–327 | MMAPLWRLNA | 11S globulin | Q84ND2 (Bertholletia excelsa) |
| B | 1145.49 | 434–442 | RLSQEEARR | 11S globulin | Q84ND2 (Bertholletia excelsa) |
| B | 2256.85 | 42–60 | QYRLEAEAGVSE VWDYTDQ | 11S globulin | Q84ND2 (Bertholletia excelsa) |
| C | 1202.46 | 184–193 | RHFFLAGNIQ | 11S globulin | Q84ND2 (Bertholletia excelsa) |
| C | 999.37 | 90–97 | LYYVTQGR | 11S globulin | Q84ND2 (Bertholletia excelsa) |
| C | 882.39 | 131–137 | QDQHQKV | 11S globulin | Q84ND2 (Bertholletia excelsa) |
| C | 1202.55 | 185–194 | HFFLAGNIQR | 11S globulin | Q84ND2 (Bertholletia excelsa) |
| D | 1165.4 | 367–376 | GETVFDDNLR | 11S globulin | Q84ND2 (Bertholletia excelsa) |
| D | 1229.54 | 422–433 | RGIPVGVLANAY | 11S globulin | Q84ND2 (Bertholletia excelsa) |
| D | 2315.75 | 49–70 | GVSEVWDYTD QQFRCAGVAAL | 11S globulin | Q84ND2 (Bertholletia excelsa) |
| Assay | Raw Material | Beverages | Byproducts | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BN 1 | WBM 1 | BM-S 1 | BM-D 2 | Cake 2 | ||||||
| DPPH | 1.72 | ±0.11 a | 0.08 | ±0.00 b | 0.07 | ±0.00 b | 1.52 | ±0.04 c | 0.27 | ±0.03 d |
| TEAC | 14.39 | ±0.46 a | 0.82 | ±0.04 b | 0.71 | ±0.03 b | 11.03 | ±0.89 c | 3.34 | ±0.15 d |
| ORAC | 10.98 | ±0.45 a | 0.61 | ±0.01 b | 0.47 | ±0.01 b | 14.18 | ±0.77 c | 6.73 | ±0.40 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasquez-Rojas, W.V.; Martín, D.; Miralles, B.; Recio, I.; Fornari, T.; Cano, M.P. Composition of Brazil Nut (Bertholletia excels HBK), Its Beverage and By-Products: A Healthy Food and Potential Source of Ingredients. Foods 2021, 10, 3007. https://doi.org/10.3390/foods10123007
Vasquez-Rojas WV, Martín D, Miralles B, Recio I, Fornari T, Cano MP. Composition of Brazil Nut (Bertholletia excels HBK), Its Beverage and By-Products: A Healthy Food and Potential Source of Ingredients. Foods. 2021; 10(12):3007. https://doi.org/10.3390/foods10123007
Chicago/Turabian StyleVasquez-Rojas, Wilson V., Diana Martín, Beatriz Miralles, Isidra Recio, Tiziana Fornari, and M. Pilar Cano. 2021. "Composition of Brazil Nut (Bertholletia excels HBK), Its Beverage and By-Products: A Healthy Food and Potential Source of Ingredients" Foods 10, no. 12: 3007. https://doi.org/10.3390/foods10123007
APA StyleVasquez-Rojas, W. V., Martín, D., Miralles, B., Recio, I., Fornari, T., & Cano, M. P. (2021). Composition of Brazil Nut (Bertholletia excels HBK), Its Beverage and By-Products: A Healthy Food and Potential Source of Ingredients. Foods, 10(12), 3007. https://doi.org/10.3390/foods10123007