Exploring the Effect of Pulsed Electric Fields on the Technological Properties of Chicken Meat
Abstract
:1. Introduction
2. Materials and Methods
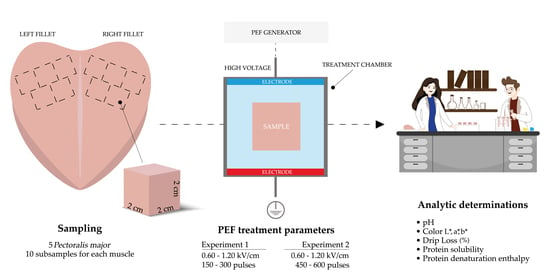
2.1. Experimental Design
2.1.1. Trial 1
2.1.2. Trial 2
2.2. Analytic Determinations
2.2.1. pH
2.2.2. Color
2.2.3. Drip Loss
2.2.4. Protein Solubility
2.2.5. Protein Denaturation Enthalpy
2.2.6. Statistical Analysis
3. Results
3.1. Experiment 1
3.2. Experiment 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Saraiva, J.A.; Raso, J.; Martin-Belloso, O.; Witrowa-Rajchert, D.; et al. Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Res. Int. 2015, 77, 773–798. [Google Scholar] [CrossRef]
- Han, Z.; Cai, M.J.; Cheng, J.H.; Sun, D.W. Effects of electric fields and electromagnetic wave on food protein structure and functionality: A review. Trends Food Sci. Technol. 2018, 75, 1–9. [Google Scholar] [CrossRef]
- Zhao, W.; Tang, Y.; Lu, L.; Chen, X.; Li, C. Review: Pulsed Electric Fields Processing of Protein-Based Foods. Food Bioprocess Technol. 2014, 7, 114–125. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.D.A. Current and future prospects for the use of pulsed electric field in the meat industry. Crit. Rev. Food Sci. Nutr. 2019, 59, 1660–1674. [Google Scholar] [CrossRef]
- Gómez, B.; Munekata, P.E.S.; Gavahian, M.; Barba, F.J.; Martí-Quijal, F.J.; Bolumar, T.; Campagnol, P.C.B.; Tomasevic, I.; Lorenzo, J.M. Application of pulsed electric fields in meat and fish processing industries: An overview. Food Res. Int. 2019, 123, 95–105. [Google Scholar] [CrossRef]
- Tylewicz, U. How does pulsed electric field work? In Pulsed Electric Fields to Obtain Healthier and Sustainable Food for Tomorrow; Barba, F.J., Parniakov, O., Wiktor, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 3–21. [Google Scholar]
- Kotnik, T.; Kramar, P.; Pucihar, G.; Miklavčič, D.; Tarek, M. Cell membrane electroporation—Part 1: The phenomenon. IEEE Electr. Insul. Mag. 2012, 28, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Toepfl, S.; Siemer, C.; Saldaña-Navarro, G.; Heinz, V. Overview of Pulsed Electric Fields Processing for Food. In Emerging Technologies for Food Processing; Elsevier: Amsterdam, The Netherlands, 2014; pp. 93–114. [Google Scholar]
- Balasa, A. Stress Response of Plants, Metabolite Production due to Pulsed Electric Fields. In Handbook of Electroporation; Springer International Publishing: New York, NY, USA, 2016; pp. 1–13. [Google Scholar]
- Zimmermann, U.; Pilwat, G.; Beckers, F.; Riemann, F. Effects of external electrical fields on cell membranes. Bioelectrochemistry Bioenerg. 1976, 3, 58–83. [Google Scholar] [CrossRef]
- Kotnik, T.; Rems, L.; Tarek, M.; Miklavcic, D. Membrane Electroporation and Electropermeabilization: Mechanisms and Models. Annu. Rev. Biophys. 2019, 48, 63–91. [Google Scholar] [CrossRef]
- Rems, L.; Viano, M.; Kasimova, M.A.; Miklavčič, D.; Tarek, M. The contribution of lipid peroxidation to membrane permeability in electropermeabilization: A molecular dynamics study. Bioelectrochemistry 2019, 125, 46–57. [Google Scholar] [CrossRef]
- Toepfl, S.; Heinz, V.; Knorr, D. Applications of pulsed electric fields technology for the food industry. In Pulsed Electric Fields Technology for the Food Industry; Raso, J., Heinz, V., Eds.; Springer Science: Berlin, Germany, 2006; pp. 197–221. [Google Scholar]
- Chauhan, O.P.; Unni, L.E. Pulsed electric field (PEF) processing of foods and its combination with electron beam processing. In Electron Beam Pasteurization and Complementary Food Processing Technologies; Shima, S., Suresh, P., Eds.; Woodhead Publishing: Cambridge, UK, 2015; pp. 157–184. [Google Scholar]
- Poojary, M.M.; Roohinejad, S.; Koubaa, M.; Barba, F.J.; Passamonti, P.; Režek Jambrak, A.; Oey, I.; Greiner, R. Impact of Pulsed Electric Fields on Enzymes. In Handbook of Electroporation; Miklavcic, D., Ed.; Springer Science: Berlin, Germany, 2016; pp. 1–21. [Google Scholar]
- Raso, J.; Frey, W.; Ferrari, G.; Pataro, G.; Knorr, D.; Teissie, J.; Miklavčič, D. Recommendations guidelines on the key information to be reported in studies of application of PEF technology in food and biotechnological processes. Innov. Food Sci. Emerg. Technol. 2016, 37, 312–321. [Google Scholar] [CrossRef] [Green Version]
- Siemer, C.; Toepfl, S.; Heinz, V. Mass Transport Improvement by PEF—Applications in the Area of Extraction and Distillation. In Distillation—Advances from Modeling to Applications; Sina, Z., Ed.; InTech: London, UK, 2012; pp. 211–232. [Google Scholar]
- Wiktor, A.; Iwaniuk, M.; Śledź, M.; Nowacka, M.; Chudoba, T.; Witrowa-Rajchert, D. Drying Kinetics of Apple Tissue Treated by Pulsed Electric Field. Dry. Technol. 2013, 31, 112–119. [Google Scholar] [CrossRef]
- Alahakoon, A.U.; Faridnia, F.; Bremer, P.J.; Silcock, P.; Oey, I. Pulsed electric fields effects on meat tissue quality and functionality. In Handbook of Electroporation; Miklavcic, D., Ed.; Springer Science: Berlin, Germany, 2017; Volume 4, pp. 2455–2475. [Google Scholar]
- OECD-FAO. Agricultural Outlook (OECD); FAO: Roman, Italy, 2020; ISBN 9789264317673. [Google Scholar]
- Baldi, G.; Soglia, F.; Petracci, M. Current Status of Poultry Meat Abnormalities. Meat Muscle Biol. 2020, 4, 1–7. [Google Scholar] [CrossRef]
- Faridnia, F.; Bekhit, A.E.D.A.; Niven, B.; Oey, I. Impact of pulsed electric fields and post-mortem vacuum ageing on beef longissimus thoracis muscles. Int. J. Food Sci. Technol. 2014, 49, 2339–2347. [Google Scholar] [CrossRef]
- Bekhit, A.E.D.A.; van de Ven, R.; Suwandy, V.; Fahri, F.; Hopkins, D.L. Effect of Pulsed Electric Field Treatment on Cold-Boned Muscles of Different Potential Tenderness. Food Bioprocess Technol. 2014, 7, 3136–3146. [Google Scholar] [CrossRef]
- Jeacocke, R.E. Continuous measurements of the pH of beef muscle in intact beef carcases. Int. J. Food Sci. Technol. 1977, 12, 375–386. [Google Scholar] [CrossRef]
- Petracci, M.; Baéza, E. Harmonization of methodology of assessment of meat quality features. World Poult. Sci. J. 2011, 67, 137–153. [Google Scholar] [CrossRef]
- Warner, R.D.; Kauffman, R.G.; Greaser, M.L. Muscle protein changes post mortem in relation to pork quality traits. Meat Sci. 1997, 45, 339–352. [Google Scholar] [CrossRef]
- Baldi, G.; Soglia, F.; Laghi, L.; Tappi, S.; Rocculi, P.; Tavaniello, S.; Prioriello, D.; Mucci, R.; Maiorano, G.; Petracci, M. Comparison of quality traits among breast meat affected by current muscle abnormalities. Food Res. Int. 2019, 115, 369–376. [Google Scholar] [CrossRef]
- Khan, A.A.; Randhawa, M.A.; Carne, A.; Mohamed Ahmed, I.A.; Barr, D.; Reid, M.; Bekhit, A.E.D.A. Quality and Nutritional Minerals in Chicken Breast Muscle Treated with Low and High Pulsed Electric Fields. Food Bioprocess Technol. 2018, 11, 122–131. [Google Scholar] [CrossRef]
- Suwandy, V.; Carne, A.; van de Ven, R.; Bekhit, A.E.D.A.; Hopkins, D.L. Effect of Repeated Pulsed Electric Field Treatment on the Quality of Cold-Boned Beef Loins and Topsides. Food Bioprocess Technol. 2015, 8, 1218–1228. [Google Scholar] [CrossRef]
- Faridnia, F.; Bremer, P.; Burritt, D.J.; Oey, I. Effect of pulsed electric fields on selected quality attributes of beef outside flat (biceps femoris). In Proceedings of 1st World Congress on Electroporation and Pulsed Electric Fields in Biology, Medicine, and Food Environmental Technologies; Springer: Singapore, 2016; Volume 53, pp. 307–310. [Google Scholar]
- Wideman, N.; O’Bryan, C.A.; Crandall, P.G. Factors affecting poultry meat colour and consumer preferences—A review. Worlds Poult. Sci. J. 2016, 72, 353–366. [Google Scholar] [CrossRef]
- O’Dowd, L.P.; Arimi, J.M.; Noci, F.; Cronin, D.A.; Lyng, J.G. An assessment of the effect of pulsed electrical fields on tenderness and selected quality attributes of post rigour beef muscle. Meat Sci. 2013, 93, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, C.; Eslami, S.; Brunton, N.P.; Arimi, J.M.; Noci, F.; Lyng, J.G. An assessment of the impact of pulsed electric fields processing factors on oxidation, color, texture, and sensory attributes of turkey breast meat. Poult. Sci. 2015, 94, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, C.; Lascorz, D.; O’Dowd, L.; Noci, F.; Arimi, J.; Lyng, J.G. Effect of Pulsed Electric Field treatments at various stages during conditioning on quality attributes of beef longissimus thoracis et lumborum muscle. Meat Sci. 2014, 99, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Barbut, S. The Science of Poultry and Meat Processing; University of Guelph: Guelph, ON, Canada, 2015; ISBN 9780889556256. [Google Scholar]
- Hughes, J.M.; Clarke, F.M.; Purslow, P.P.; Warner, R.D. Meat color is determined not only by chromatic heme pigments but also by the physical structure and achromatic light scattering properties of the muscle. Compr. Rev. Food Sci. Food Saf. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowker, B. Developments in our understanding of water-holding capacity. In Poultry Quality Evaluation: Quality Attributes and Consumer Values; Berri, C., Petracci, M., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 77–114. [Google Scholar]
- Gudmundsson, M.; Hafsteinsson, H. Effect of electric field pulses on microstructure of muscle foods and roes. Trends Food Sci. Technol. 2001, 12, 122–128. [Google Scholar] [CrossRef]
- Saulis, G. Electroporation of cell membranes: The fundamental effects of pulsed electric fields in food processing. Food Eng. Rev. 2010, 2, 52–73. [Google Scholar] [CrossRef]
- Swatland, H. Meat Science: An Introductory Text; Cabi Publishing: Wallingford, UK, 2000. [Google Scholar]
- Yeom, H.W.; Zhang, Q.H.; Dunne, C.P. Inactivation of papain by pulsed electric fields in a continuous system. Food Chem. 1999, 67, 53–59. [Google Scholar] [CrossRef]
- Mirmoghtadaie, L.; Shojaee Aliabadi, S.; Hosseini, S.M. Recent approaches in physical modification of protein functionality. Food Chem. 2016, 199, 619–627. [Google Scholar] [CrossRef]
- Dong, M.; Xu, Y.; Zhang, Y.; Han, M.; Wang, P.; Xu, X.; Zhou, G. Physicochemical and structural properties of myofibrillar proteins isolated from pale, soft, exudative (PSE)-like chicken breast meat: Effects of pulsed electric field (PEF). Innov. Food Sci. Emerg. Technol. 2020, 59, 102277. [Google Scholar] [CrossRef]
- Bertram, C.H.; Kristensen, M.; Østdal, H.; Baron, C.P.; Young, J.F.; Jørgen Andersen, H. Does Oxidation Affect the Water Functionality of Myofibrillar Proteins? J. Agric. Food Chem. 2007, 55, 2342–2348. [Google Scholar] [CrossRef] [PubMed]

| Treatments (T) 1 | Electric Field Strength (kV/cm) | Energy Input 2 (kJ/kg) | Frequency (Hz) | Treatment Time (s) | Pulse Number 3 | Pulse Width (µs) |
|---|---|---|---|---|---|---|
| Control | 0 | 0 | 0 | 0 | 0 | 0 |
| T1 | 0.60 ± 0.01 | 0.15 ± 0.01 | 50 | 3 | 150 | 20 |
| T2 | 1.20 ± 0.02 | 0.59 ± 0.01 | 50 | 3 | 150 | 20 |
| T3 | 0.60 ± 0.02 | 0.31 ± 0.01 | 50 | 6 | 300 | 20 |
| T4 | 1.20 ± 0.03 | 1.19 ± 0.04 | 50 | 6 | 300 | 20 |
| Treatments (T) 1 | Electric Field Strength (kV/cm) | Energy Input 2 (kJ/kg) | Frequency (Hz) | Total Treatment Time (s) | Pulse Number 3 | Pulse Width (µs) |
|---|---|---|---|---|---|---|
| Control | 0 | 0 | 0 | 0 | 0 | 0 |
| T5 | 0.60 ± 0.02 | 0.45 ± 0.01 | 50 | 9 | 450 | 20 |
| T6 | 1.20 ± 0.03 | 1.77 ± 0.05 | 50 | 9 | 450 | 20 |
| T7 | 0.60 ± 0.02 | 0.66 ± 0.02 | 50 | 12 | 600 | 20 |
| T8 | 1.20 ± 0.03 | 2.42 ± 0.09 | 50 | 12 | 600 | 20 |
| Parameters | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment (T) | Electric Field Strength 1 | Pulses no | n | pH | ∆L* | ∆a* | ∆b* | Drip Loss (%) | ||||
| 24 h | 48 h | 72 h | 96 h | Total | ||||||||
| Control | - | - | 5 | 5.95 ± 0.07 | - | - | - | 3.95 a ± 0.54 | 0.79 ± 0.49 | 1.56 ± 0.55 | 1.19 ± 0.24 | 7.30 a ± 1.66 |
| T1 | Low | 150 | 5 | 5.90 ± 0.08 | 2.34 ab ± 0.71 | −0.40 ± 0.26 | 0.06 ± 0.69 | 3.42 ab ± 0.45 | 0.57 ± 0.28 | 1.33 ± 0.34 | 1.02 ± 0.28 | 6.21 ab ± 1.14 |
| T2 | High | 150 | 5 | 5.93 ± 0.07 | −0.46 bc ± 0.41 | −0.44 ± 0.27 | −0.56 ± 1.09 | 2.82 bc ± 0.41 | 0.50 ± 0.25 | 1.19 ± 0.40 | 1.00 ± 0.30 | 5.41 ab ± 1.25 |
| T3 | Low | 300 | 5 | 5.95 ± 0.06 | 3.38 a ± 2.84 | −0.22 ± 0.17 | 0.85 ± 1.18 | 2.25 cd ± 0.28 | 0.52 ± 0.20 | 1.10 ± 0.35 | 1.01 ± 0.37 | 4.79 b ± 1.05 |
| T4 | High | 300 | 5 | 5.93 ± 0.06 | −1.44c ± 1.18 | −0.47 ± 0.48 | −0.39 ± 1.61 | 1.85 d ± 0.26 | 0.65 ± 0.17 | 1.04 ± 0.25 | 0.99 ± 0.26 | 4.46 b ± 0.84 |
| p-Value | n.s. | ** | n.s. | n.s. | *** | n.s. | n.s. | n.s. | * | |||
| Planned contrast 2 | ||||||||||||
| Control | 5 | 5.95 ± 0.07 | - | - | - | 3.95 ± 0.54 | 0.79 ± 0.49 | 1.56 ± 0.55 | 1.19 ± 0.24 | 7.30 ± 1.66 | ||
| Treated | 20 | 5.93 ± 0.07 | - | - | - | 2.58 ± 0.69 | 0.56 ± 0.22 | 1.17± 0.33 | 1.01 ± 0.28 | 5.22 ± 1.20 | ||
| p-Value | n.s. | - | - | - | *** | n.s. | n.s. | n.s. | ** | |||
| Low electric field strength | 10 | 5.93 ± 0.07 | 2.87 ± 1.03 | −0.32 ± 0.23 | 0.46 ± 1.01 | 2.83 ± 0.71 | 0.54 ± 0.23 | 1.21 ± 0.35 | 1.02 ± 0.31 | 5.50 ± 1.28 | ||
| High electric field strength | 10 | 5.93 ± 0.06 | −1.01 ± 1.84 | −0.45 ± 0.38 | −0.46 ± 0.33 | 2.33 ± 0.60 | 0.58 ± 0.22 | 1.12 ± 0.33 | 1.00 ± 0.26 | 4.94 ± 1.12 | ||
| p-Value | n.s. | ** | n.s. | n.s. | * | n.s. | n.s. | n.s. | n.s. | |||
| 150 pulses | 10 | 5.91 ± 0.07 | 1.10 ± 0.79 | −0.42 ± 0.25 | −0.21 ± 0.89 | 3.12 ± 0.52 | 0.53 ± 0.25 | 1.26 ± 0.36 | 1.01 ± 0.28 | 5.81 ± 1.20 | ||
| 300 pulses | 10 | 5.94 ± 0.06 | 0.97 ± 0.49 | −0.34 ± 0.36 | 0.23 ± 0.49 | 2.05 ± 0.33 | 0.58 ± 0.19 | 1.07 ± 0.29 | 1.00 ± 0.30 | 4.62 ± 0.91 | ||
| p-Value | n.s. | n.s. | n.s. | n.s. | *** | n.s. | n.s. | n.s. | * | |||
| Parameters | |||||||
|---|---|---|---|---|---|---|---|
| Treatment (T) | Electric Field Strength 1 | Pulses no | n | Protein Solubility (mg/g) | Protein Denaturation Enthalpy (ΔH, J/g) | ||
| Myofibrillar | Sarcoplasmic | Total | |||||
| Control | - | - | 5 | 206.2 ± 32.3 | 164.8 ± 88.5 | 370.9 ± 103.9 | 2.66 ± 0.44 |
| T1 | Low | 150 | 5 | 209.5 ± 47.3 | 161.1 ± 11.0 | 370.6 ± 52.3 | 2.90 ± 0.26 |
| T2 | High | 150 | 5 | 229.6 ± 31.3 | 168.3 ± 5.60 | 397.9 ± 30.7 | 3.05 ± 0.27 |
| T3 | Low | 300 | 5 | 229.5 ± 32.5 | 163.6 ± 26.5 | 393.1 ± 26.4 | 2.84 ± 0.20 |
| T4 | High | 300 | 5 | 191.5 ± 19.3 | 204.5 ± 70.2 | 396.0 ± 33.3 | 2.54 ± 0.34 |
| p-Value | n.s. | n.s. | n.s. | n.s. | |||
| Planned contrast 2 | |||||||
| Control | 5 | 206.2 ± 27.4 | 164.8 ± 15.6 | 370.9 ± 33.6 | 2.66 ± 0.44 | ||
| Treated | 20 | 215.0 ± 35.2 | 174.4 ± 39.3 | 378.6 ± 38.7 | 2.83 ± 0.31 | ||
| p-Value | n.s. | n.s. | n.s. | n.s. | |||
| Low electric field strength | 10 | 219.5 ± 39.6 | 162.3 ± 19.2 | 381.8 ± 40.8 | 2.87 ± 0.22 | ||
| High electric field strength | 10 | 210.6 ± 31.7 | 186.4 ± 50.7 | 375.5 ± 38.4 | 2.80 ± 0.39 | ||
| p-Value | n.s. | n.s. | n.s. | n.s. | |||
| 150 pulses | 10 | 219.5 ± 39.2 | 164.7 ± 9.06 | 384.2 ± 42.9 | 2.98 ± 0.26 | ||
| 300 pulses | 10 | 210.5 ± 32.2 | 184.1 ± 54.5 | 373.0 ± 35.4 | 2.69 ± 0.30 | ||
| p-Value | n.s. | n.s. | n.s. | n.s. | |||
| Parameters | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment (T) | Electric Field Strength 1 | Pulses no | n | pH | ∆L* | ∆a* | ∆b* | Drip Loss (%) | ||||
| 24 h | 48 h | 72 h | 96 h | Total | ||||||||
| Control | - | - | 5 | 5.95 ± 0.13 | - | - | - | 3.03 a ± 0.52 | 0.99 ± 0.28 | 1.12 ± 0.40 | 0.92 ± 0.32 | 6.08 ab ± 1.15 |
| T5 | Low | 450 | 5 | 5.93 ± 0.12 | 0.03 b ± 0.84 | −0.79 ± 0.67 | −1.79 b ± 0.72 | 3.06 a ± 0.12 | 1.06 ± 0.19 | 1.11 ± 0.28 | 0.97 ± 0.15 | 6.20 a ± 0.47 |
| T6 | High | 450 | 5 | 5.96 ± 0.11 | 0.64 ab ± 2.15 | −0.26 ± 0.44 | 0.27 a ± 0.97 | 2.67 a ± 0.34 | 1.06 ± 0.16 | 0.94 ± 0.10 | 0.94 ± 0.09 | 5.60 ab ± 0.55 |
| T7 | Low | 600 | 5 | 5.95 ± 0.14 | 2.60 a ± 1.35 | −0.18 ± 0.07 | 0.80 a ± 0.83 | 2.17 b ± 0.14 | 0.99 ± 0.19 | 0.93 ± 0.10 | 0.91 ± 0.11 | 5.00 b ± 0.37 |
| T8 | High | 600 | 5 | 5.94 ± 0.14 | 2.34 a ± 1.41 | −0.60 ± 0.49 | 1.30 a ± 0.29 | 1.81 b ± 0.16 | 1.09 ± 0.35 | 0.88 ± 0.10 | 0.93 ± 0.11 | 4.71b ± 0.58 |
| p-Value | n.s. | * | n.s. | *** | *** | n.s. | n.s. | n.s. | ** | |||
| Planned contrast 2 | ||||||||||||
| Control | 5 | 5.95 ± 0.12 | - | - | - | 3.03 ± 0.57 | 0.99 ± 0.23 | 1.12 ± 0.23 | 0.92 ± 0.16 | 6.08 ± 0.86 | ||
| Treated | 20 | 5.95 ± 0.12 | - | - | - | 2.42 ± 0.52 | 1.05 ± 0.22 | 0.97 ± 0.17 | 0.94 ± 0.11 | 5.38 ± 0.74 | ||
| p-Value | n.s. | - | - | - | *** | n.s. | n.s. | n.s. | * | |||
| Low electric field strength | 10 | 5.94 ± 0.12 | 1.32 ± 1.72 | −0.48 ± 0.55 | −0.50 ± 1.55 | 2.61 ± 0.49 | 1.03 ± 0.19 | 1.02 ± 0.22 | 0.94 ± 0.13 | 5.60 ± 0.75 | ||
| High electric field strength | 10 | 5.95 ± 0.12 | 1.49 ± 1.94 | −0.43 ± 0.47 | 0.79 ± 0.86 | 2.24 ± 0.52 | 1.08 ± 0.26 | 0.91 ± 0.10 | 0.93 ± 0.09 | 5.16 ± 0.71 | ||
| p-Value | n.s. | n.s. | n.s. | ** | * | n.s. | n.s. | n.s. | n.s. | |||
| 450 pulses | 10 | 5.95 ± 0.11 | 0.33 ± 1.58 | −0.53 ± 0.60 | −0.76 ± 1.35 | 2.86 ± 0.32 | 1.06 ±0.17 | 1.02 ± 0.22 | 0.95 ± 0.12 | 5.90 ± 0.58 | ||
| 600 pulses | 10 | 5.95 ± 0.13 | 2.47 ± 1.30 | −0.39 ± 0.40 | 1.05 ± 0.65 | 1.99 ± 0.24 | 1.04 ± 0.27 | 0.91 ± 0.10 | 0.92 ± 0.11 | 4.86 ± 0.48 | ||
| p-Value | n.s. | ** | n.s. | *** | *** | n.s. | n.s. | n.s. | ** | |||
| . | Parameters | ||||||
|---|---|---|---|---|---|---|---|
| Treatment (T) | Electric Field Strength 1 | Pulses no | n | Protein Solubility (mg/g) | Protein Denaturation Enthalpy (ΔH, J/g) | ||
| Myofibrillar | Sarcoplasmic | Total | |||||
| Control | - | - | 5 | 180.3 ± 38.4 | 160.8 ab ± 7.62 | 341.1 ± 43.9 | 2.95 ± 0.35 |
| T5 | Low | 450 | 5 | 150.5 ± 34.4 | 155.9 ab ± 8.94 | 306.4 ± 32.2 | 3.21 ± 0.29 |
| T6 | High | 450 | 5 | 160.3 ± 46.7 | 150.1 b ± 10.3 | 310.4 ± 49.2 | 3.36 ± 0.74 |
| T7 | Low | 600 | 5 | 143.1 ± 37.8 | 169.3 ab ± 15.2 | 312.4 ± 24.4 | 3.32 ± 0.25 |
| T8 | High | 600 | 5 | 144.8 ± 24.3 | 175.9 a ± 20.4 | 304.7 ± 37.9 | 3.30 ± 0.16 |
| p-Value | n.s. | * | n.s. | n.s. | |||
| Planned contrast 2 | |||||||
| Control | 5 | 180.3 ± 38.4 | 160.8 ± 7.62 | 341.1 ± 43.9 | 2.95 ± 0.35 | ||
| Treated | 20 | 149.7 ± 34.4 | 162.8 ± 16.9 | 312.5 ± 34.2 | 3.30 ± 0.41 | ||
| p-Value | n.s. | n.s. | n.s. | n.s. | |||
| Low electric field strength | 10 | 146.8 ± 34.3 | 162.6 ± 13.7 | 309.4 ± 27.1 | 3.26 ± 0.25 | ||
| High electric field strength | 10 | 152.5 ± 36.1 | 163.0 ± 20.4 | 315.5 ± 41.6 | 3.34 ± 0.53 | ||
| p-Value | n.s. | n.s. | n.s. | n.s. | |||
| 450 pulses | 10 | 155.4 ± 34.4 | 153.0 ± 16.9 | 308.4 ± 34.2 | 3.30 ± 0.41 | ||
| 600 pulses | 10 | 143.9 ± 39.0 | 172.6 ± 9.56 | 316.5 ± 39.3 | 3.31 ± 0.56 | ||
| p-Value | n.s | ** | n.s. | n.s. | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldi, G.; D’Elia, F.; Soglia, F.; Tappi, S.; Petracci, M.; Rocculi, P. Exploring the Effect of Pulsed Electric Fields on the Technological Properties of Chicken Meat. Foods 2021, 10, 241. https://doi.org/10.3390/foods10020241
Baldi G, D’Elia F, Soglia F, Tappi S, Petracci M, Rocculi P. Exploring the Effect of Pulsed Electric Fields on the Technological Properties of Chicken Meat. Foods. 2021; 10(2):241. https://doi.org/10.3390/foods10020241
Chicago/Turabian StyleBaldi, Giulia, Fabio D’Elia, Francesca Soglia, Silvia Tappi, Massimiliano Petracci, and Pietro Rocculi. 2021. "Exploring the Effect of Pulsed Electric Fields on the Technological Properties of Chicken Meat" Foods 10, no. 2: 241. https://doi.org/10.3390/foods10020241
APA StyleBaldi, G., D’Elia, F., Soglia, F., Tappi, S., Petracci, M., & Rocculi, P. (2021). Exploring the Effect of Pulsed Electric Fields on the Technological Properties of Chicken Meat. Foods, 10(2), 241. https://doi.org/10.3390/foods10020241