Physico-Chemical Characteristics and In Vitro Gastro-Small Intestinal Digestion of New Zealand Ryegrass Proteins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemicals
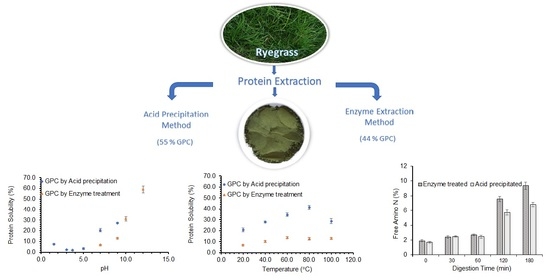
2.2. Preparation of Grass Protein Concentrate Using Acid Precipitation
2.3. Preparation of Grass Protein Concentrate Using Enzyme Treatment
2.4. Proximate Analysis
2.5. Differential Scanning Calorimetry
2.6. Effect of pH and Temperature on Solubility of Grass Protein Concentrate
2.7. Determination of Gastro-Small Intestinal In Vitro Protein Digestibility
2.7.1. In Vitro Gastro-Small Intestinal Digestion
2.7.2. Soluble N and Free Amino N Contents
3. Results & Discussion
3.1. Protein Extraction Processes, GPC Yield (%) and Composition
3.2. Thermal Stability of the Grass Protein Concentrate
3.3. Solubility of GPC
3.3.1. Effect of pH on Protein Solubility
3.3.2. Effect of Temperature on Protein Solubility
3.3.3. Effect of Salt Concentration on Protein Solubility
3.4. In Vitro Protein Digestibility of GPC
3.4.1. Soluble Nitrogen Content during Digestion
3.4.2. Free Amino Nitrogen Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoehnel, A.; Axel, C.; Bez, J.; Arendt, E.K.; Zannini, E. Comparative analysis of plant-based high-protein ingredients and their impact on quality of high-protein bread. J. Cereal Sci. 2019, 89, 102816. [Google Scholar] [CrossRef]
- Barbeau, W.E.; Kinsella, J.E. Ribulose bisphosphate carboxylase/oxygenase (rubisco) from green leaves-potential as a food protein. Food Rev. Int. 1988, 4, 93–127. [Google Scholar] [CrossRef]
- Solati, Z.; Manevski, K.; Jørgensen, U.; Labouriau, R.; Shahbazi, S.; Lærke, P.E. Crude protein yield and theoretical extractable true protein of potential biorefinery feedstocks. Ind. Crops Prod. 2018, 115, 214–226. [Google Scholar] [CrossRef]
- Hayati Zeidanloo, M.; Ahmadzadeh Ghavidel, R.; Ghiafeh Davoodi, M.; Arianfar, A. Functional properties of Grass pea protein concentrates prepared using various precipitation methods. J. Food Sci. Technol. 2019, 56, 4799–4808. [Google Scholar] [CrossRef]
- Di Stefano, E.; Agyei, D.; Njoku, E.N.; Udenigwe, C.C. Plant RuBisCo: An underutilized protein for food applications. J. Am. Oil Chem. Soc. 2018, 95, 1063–1074. [Google Scholar] [CrossRef]
- Sheen, S.J.; Sheen, V.L. Functional properties of fraction 1 protein from tobacco leaf. J. Agric. Food Chem. 1985, 33, 79–83. [Google Scholar] [CrossRef]
- Amer, B.; Juul, L.; Møller, A.H.; Møller, H.S.; Dalsgaard, T.K. Improved solubility of proteins from white and red clover—Inhibition of redox enzymes. Int. J. Food Sci. Technol. 2021, 56, 302–311. [Google Scholar] [CrossRef]
- Pojić, M.; Mišan, A.; Tiwari, B. Eco-innovative technologies for extraction of proteins for human consumption from renewable protein sources of plant origin. Trends Food Sci. Technol. 2018, 75, 93–104. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.; Rahman, M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Wu, K.; Ju, T.; Deng, Y.; Xi, J. Mechanochemical assisted extraction: A novel, efficient, eco-friendly technology. Trends Food Sci. Technol. 2017, 66, 166–175. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Selvamuthukumaran, M.; Shi, J. Recent advances in extraction of antioxidants from plant by-products processing industries. Food Qual. Saf. 2017, 1, 61–81. [Google Scholar] [CrossRef]
- Shen, L.; Wang, X.; Wang, Z.; Wu, Y.; Chen, J. Studies on tea protein extraction using alkaline and enzyme methods. Food Chem. 2008, 107, 929–938. [Google Scholar] [CrossRef]
- Agriseeds. Trojan Perennial Ryegrass; Barenbrug: Christchurch, New Zealand, 2013. [Google Scholar]
- Joint FAO/WHO Food Standards Programme Codex Alimentarius Commission. General standard for vegetable protein products (VPP) CXS 174-1989. In Codex Alimentarius/Joint FAO/WHO Food Standards Programme, Codex Alimentarius Commission; Food and Agriculture Organization of the United Nations, World Health Organization: Rome, Italy, 2019; Adopted in 1989, Amended in 2019. [Google Scholar]
- de Figueiredo, V.R.G.; Yamashita, F.; Vanzela, A.L.L.; Ida, E.I.; Kurozawa, L.E. Action of multi-enzyme complex on protein extraction to obtain a protein concentrate from okara. J. Food Sci. Technol. 2018, 55, 1508–1517. [Google Scholar] [CrossRef]
- Zhang, C.; Sanders, J.P.M.; Bruins, M.E. Critical parameters in cost-effective alkaline extraction for high protein yield from leaves. Biomass Bioenergy 2014, 67, 466–472. [Google Scholar] [CrossRef]
- Horwitz, W. (Ed.) Official Methods of Analysis of AOAC International. Volume I, Agricultural Chemicals, Contaminants, Drugs; AOAC International: Gaithersburg, MD, USA, 1997. [Google Scholar]
- Jiang, B.; Tsao, R.; Li, Y.; Miao, M. Food Safety: Food Analysis Technologies/Techniques; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Singh, J.; McCarthy, O.J.; Singh, H.; Moughan, P.J.; Kaur, L. Morphological, thermal and rheological characterization of starch isolated from New Zealand Kamo Kamo (Cucurbita pepo) fruit—A novel source. Carbohydr. Polym. 2007, 67, 233–244. [Google Scholar] [CrossRef]
- Ahmad, S.; Nema, P.; Bashir, K. Effect of different drying techniques on physicochemical, thermal, and functional properties of seera. Dry. Technol. 2018, 36, 1284–1291. [Google Scholar] [CrossRef]
- Bolontrade, A.J.; Scilingo, A.A.; Añón, M.C. Amaranth proteins foaming properties: Adsorption kinetics and foam formation—Part 1. Colloids Surf. B Biointerfaces 2013, 105, 319–327. [Google Scholar] [CrossRef]
- Pelegrine, D.; Gasparetto, C. Whey proteins solubility as function of temperature and pH. LWT Food Sci. Technol. 2005, 38, 77–80. [Google Scholar] [CrossRef]
- Hamm, R.; Deatherage, F.E. Changes in hydration, solubility and charges of muscle proteins during heating of meat a. J. Food Sci. 1960, 25, 587–610. [Google Scholar] [CrossRef]
- Yildiz, G. Effect of pH-shifting method on solubility and emulsifying properties of soy protein concentrate. Harran Tarım Gıda Bilimleri Derg. 2018, 23, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Chian, F.M.; Kaur, L.; Oey, I.; Astruc, T.; Hodgkinson, S.; Boland, M. Effect of Pulsed Electric Fields (PEF) on the ultrastructure and in vitro protein digestibility of bovine longissimus thoracis. LWT 2019, 103, 253–259. [Google Scholar] [CrossRef]
- Moore, S. Amino acid analysis: Aqueous dimethyl sulfoxide as solvent for the ninhydrin reaction. J. Biol. Chem. 1968, 243, 6281–6283. [Google Scholar] [CrossRef]
- Kaur, L.; Astruc, T.; Vénien, A.; Loison, O.; Cui, J.; Irastorza, M.; Boland, M. High pressure processing of meat: Effects on ultrastructure and protein digestibility. Food Funct. 2016, 7, 2389–2397. [Google Scholar] [CrossRef]
- Hanmoungjai, P.; Pyle, D.L.; Niranjan, K. Enzyme-assisted water-extraction of oil and protein from rice bran. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2002, 77, 771–776. [Google Scholar] [CrossRef]
- Cheng, X.; Bi, L.; Zhao, Z.; Chen, Y. Advances in enzyme assisted extraction of natural products. In Proceedings of the 3rd International Conference on Material, Mechanical and Manufacturing Engineering (IC3ME 2015), Guangzhou, China, 27–28 June 2015. [Google Scholar]
- Healtheries, N.Z. Spirulina: Typical Nutrient Analysis. Available online: https://healtheries.co.nz/product/spirulina-powder (accessed on 12 January 2021).
- Farkas, J.; Mohácsi-Farkas, C. Application of differential scanning calorimetry in food research and food quality assurance. J. Therm. Anal. Calorim. 1996, 47, 1787–1803. [Google Scholar] [CrossRef]
- Lamsal, B.; Koegel, R.; Gunasekaran, S. Some physicochemical and functional properties of alfalfa soluble leaf proteins. LWT-Food Sci. Technol. 2007, 40, 1520–1526. [Google Scholar] [CrossRef]
- Béghin, V.; Bizot, H.; Audebrand, M.; Lefebvre, J.; Libouga, D.G.; Douillard, R. Differential scanning calorimetric studies of the effects of ions and pH on ribulose 1,5-bisphosphate carboxylase/oxygenase. Int. J. Biol. Macromol. 1993, 15, 195–200. [Google Scholar] [CrossRef]
- Burova, T.; Soshinsky, A.; Danilenko, A.; Antonov, Y.A.; Grinberg, V.Y.; Tolstoguzov, V. Conformation stability of ribulosodiphosphatecarboxylase of alfalfa green leaves according to the data of differential scanning microcalorimetry. Biofizika 1989, 34, 545–549. [Google Scholar]
- Tomimatsu, Y. Macromolecular properties and subunit interactions of ribulose-1,5-bisphosphate carboxylase from alfalfa. Biochim. Biophys. Acta Protein Struct. 1980, 622, 85–93. [Google Scholar] [CrossRef]
- Privalov, P.L.; Potekhin, S.A. Scanning microcalorimetry in studying temperature-induced changes in proteins. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1986; Volume 131, pp. 4–51. [Google Scholar]
- Zayas, J.F. Solubility of proteins. In Functionality of Proteins in Food; Springer: Berlin/Heidelberg, Germany, 1997; pp. 6–75. [Google Scholar]
- Damodaran, S.; Parkin, K.L.; Fennema, O.R. Fennema’s Food Chemistry, 4th ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Knuckles, B.E.; Kohler, G.O. Functional properties of edible protein concentrates from alfalfa. J. Agric. Food Chem. 1982, 30, 748–752. [Google Scholar] [CrossRef]
- Xue, L.; Ren, H.; Li, S.; Gao, M.; Shi, S.; Chang, E.; Wei, Y.; Yao, X.; Jiang, Z.; Liu, J. Comparative proteomic analysis in Miscanthus sinensis exposed to antimony stress. Environ. Pollut. 2015, 201, 150–160. [Google Scholar] [CrossRef]
- Betschart, A.A. Nitrogen solubility of alfalfa protein concentrate as influenced by various factors. J. Food Sci. 1974, 39, 1110–1115. [Google Scholar] [CrossRef]
- Miller, R.E.; De Fremery, D.; Bickoff, E.; Kohler, G.O. Soluble protein concentrate from alfalfa by low-temperature acid precipitation. J. Agric. Food Chem. 1975, 23, 1177–1179. [Google Scholar] [CrossRef]
- Wang, J.; Kinsella, J. Functional properties of novel proteins: Alfalfa leaf protein. J. Food Sci. 1976, 41, 286–292. [Google Scholar] [CrossRef]
- Machado, F.F.; Coimbra, J.S.; Rojas, E.E.G.; Minim, L.A.; Oliveira, F.C.; Rita de Cássia, S.S. Solubility and density of egg white proteins: Effect of pH and saline concentration. LWT Food Sci. Technol. 2007, 40, 1304–1307. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, B.; Liu, Y.; Xiong, Y.L. Interfacial structural role of pH-shifting processed pea protein in the oxidative stability of oil/water emulsions. J. Agric. Food Chem. 2014, 62, 1683–1691. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, J.; Xiong, Y.L. Structural and emulsifying properties of soy protein isolate subjected to acid and alkaline pH-shifting processes. J. Agric. Food Chem. 2009, 57, 7576–7583. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.; Can Karaca, A.; Tyler, R.; Nickerson, M. Pea protein isolates: Structure, extraction, and functionality. Food Rev. Int. 2018, 34, 126–147. [Google Scholar] [CrossRef]
- Mu, T.H.; Tan, S.S.; Chen, J.W.; Xue, Y.L. Effect of pH and NaCl/CaCl2 on the solubility and emulsifying properties of sweet potato protein. J. Sci. Food Agric. 2009, 89, 337–342. [Google Scholar] [CrossRef]
- Aderinola, T.A.; Alashi, A.M.; Nwachukwu, I.D.; Fagbemi, T.N.; Enujiugha, V.N.; Aluko, R.E. In vitro digestibility, structural and functional properties of Moringa oleifera seed proteins. Food Hydrocoll. 2020, 101, 105574. [Google Scholar] [CrossRef]
- Eggum, B.O. The influence of dietary fibre on protein digestion and utilization in monogastrics. Arch. Für Tierernahr. 1995, 48, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Kritchevsky, D. Dietary Fiber. Annu. Rev. Nutr. 1988, 8, 301–328. [Google Scholar] [CrossRef] [PubMed]
- Joehnke, M.S.; Sørensen, S.; Bjergegaard, C.; Markedal, K.E.; Sørensen, J.C. Effect of dietary fibre fractions on in vitro digestibility of rapeseed napin proteins. Pol. J. Food Nutr. Sci. 2018, 68, 335–345. [Google Scholar] [CrossRef]








| Component | Proportion (% or mg/100 g) |
|---|---|
| Major components (%) | |
| Moisture | 3.77% |
| Ash | 2.53% |
| Protein | 54.69% |
| Fat | 8.45% |
| Carbohydrate | 26.61% |
| Insoluble Dietary Fibre | 18.40% |
| Soluble Dietary Fibre | 3.60% |
| Minerals (mg/100 g) | |
| Calcium | 143 mg/100 g |
| Magnesium | 95 mg/100 g |
| Potassium | 320 mg/100 g |
| Sodium | 121 mg/100 g |
| Iron | 40 mg/100 g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, L.; Lamsar, H.; López, I.F.; Filippi, M.; Ong Shu Min, D.; Ah-Sing, K.; Singh, J. Physico-Chemical Characteristics and In Vitro Gastro-Small Intestinal Digestion of New Zealand Ryegrass Proteins. Foods 2021, 10, 331. https://doi.org/10.3390/foods10020331
Kaur L, Lamsar H, López IF, Filippi M, Ong Shu Min D, Ah-Sing K, Singh J. Physico-Chemical Characteristics and In Vitro Gastro-Small Intestinal Digestion of New Zealand Ryegrass Proteins. Foods. 2021; 10(2):331. https://doi.org/10.3390/foods10020331
Chicago/Turabian StyleKaur, Lovedeep, Harmandeepsingh Lamsar, Ignacio F. López, Manon Filippi, Dayna Ong Shu Min, Kévin Ah-Sing, and Jaspreet Singh. 2021. "Physico-Chemical Characteristics and In Vitro Gastro-Small Intestinal Digestion of New Zealand Ryegrass Proteins" Foods 10, no. 2: 331. https://doi.org/10.3390/foods10020331
APA StyleKaur, L., Lamsar, H., López, I. F., Filippi, M., Ong Shu Min, D., Ah-Sing, K., & Singh, J. (2021). Physico-Chemical Characteristics and In Vitro Gastro-Small Intestinal Digestion of New Zealand Ryegrass Proteins. Foods, 10(2), 331. https://doi.org/10.3390/foods10020331