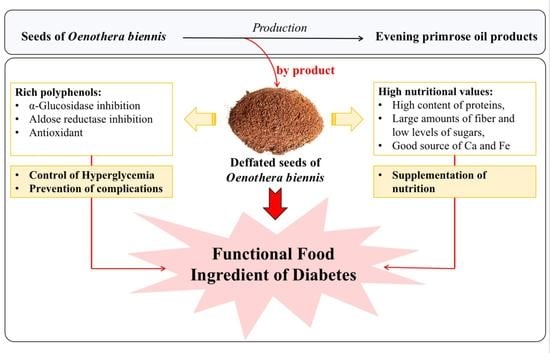
Defatted Seeds of Oenothera biennis as a Potential Functional Food Ingredient for Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Samples
2.3. Macronutrients, Energy, and Sugars
2.4. Amino Acids
2.5. Minerals
2.6. Tocopherols
2.7. Organic Acids
2.8. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.9. Characterization of Polyphenols by Liquid Chromatography Coupled with Electrospray Ionization–Quadrupole Time of Flight–Mass Spectrometry Analysis (LC–ESI–QTOF/MS)
2.10. Antioxidant
2.11. α-Glucosidase Inhibition Assay
2.12. Rat Lens Aldose Reductase Inhibition Assay
2.13. Animal Care
2.14. Statistical Analysis
3. Results and Discussion
3.1. α-Glucosidase Inhibition, Aldose Reductase Inhibition, and Antioxidant Capacity of DSOB
3.2. Polyphenol Composition of DSOB
3.3. Nutritional Value of DSOB
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federations. Diabetes Atlas. Available online: https://www.diabetesatlas.org/en/resources/ (accessed on 20 December 2019).
- Veeresham, C.; Rama Rao, A.; Asres, K. Aldose reductase inhibitors of plant origin. Phyther. Res. 2014, 28, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Gleissner, C.A.; Galkina, E.; Nadler, J.L.; Ley, K. Mechanisms by which diabetes increases cardiovascular disease. Drug Discov. Today. Dis. Mech. 2007, 4, 131–140. [Google Scholar] [CrossRef] [Green Version]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- Parkman, H.P.; Yates, K.P.; Hasler, W.L.; Nguyan, L.; Pasricha, P.J.; Snape, W.J.; Farrugia, G.; Calles, J.; Koch, K.L.; Abell, T.L.; et al. Dietary intake and nutritional deficiencies in patients with diabetic or idiopathic gastroparesis. Gastroenterology 2011, 141, 486–498. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Hwang, S.H.; Lee, S.Y.; Lim, S.S. Fermentation of purple Jerusalem artichoke extract to improve the α-glucosidase inhibitory effect in vitro and ameliorate blood glucose in db/db mice. Nutr. Res. Pract. 2016, 10, 282–287. [Google Scholar] [CrossRef] [Green Version]
- Kousaxidis, A.; Petrou, A.; Lavrentaki, V.; Fesatidou, M.; Nicolaou, I.; Geronikaki, A. Aldose reductase and protein tyrosine phosphatase 1B inhibitors as a promising therapeutic approach for diabetes mellitus. Eur. J. Med. Chem. 2020, 207, 112742. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular mechanisms linking oxidative stress and diabetes mellitus. Oxid. Med. Cell. Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, A.A.; Hastings, A.P.; Johnson, M.T.J.; Maron, J.L.; Salminen, J.P. Insect herbivores drive real-time ecological and evolutionary change in plant populations. Science 2012, 338, 113–116. [Google Scholar] [CrossRef]
- Pan, F.; Li, Y.; Luo, X.; Wang, X.; Wang, C.; Wen, B.; Guan, X.; Xu, Y.; Liu, B. Effect of the chemical refining process on composition and oxidative stability of evening primrose oil. J. Food Process. Pres. 2020, 44, e14800. [Google Scholar] [CrossRef]
- Ghasemnezhad, A.; Honermeier, B. Yield, oil constituents, and protein content of evening primrose (Oenothera biennis L.) seeds depending on harvest time, harvest method and nitrogen application. Ind. Crop Prod. 2008, 28, 17–23. [Google Scholar] [CrossRef]
- Kiss, A.K.; Naruszewicz, M. Polyphenolic compounds characterization and reactive nitrogen species scavenging capacity of Oenothera paradoxa defatted seed extracts. Food Chem. 2012, 131, 485–492. [Google Scholar] [CrossRef]
- Kiss, A.K.; Derwińska, M.; Granica, S. Quantitative analysis of biologically active polyphenols in evening primrose (Oenothera paradoxa) seeds aqueous extracts. Pol. J. Food Nutr. Sci. 2011, 61, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Pająk, P.; Socha, R.; Broniek, J.; Królikowska, K.; Fortuna, T. Antioxidant properties, phenolic and mineral composition of germinated chia, golden flax, evening primrose, phacelia and fenugreek. Food Chem. 2019, 275, 69–76. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Barros, L.; Pereira, E.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Bioactivity and chemical characterization in hydrophilic and lipophilic compounds of Chenopodium ambrosioides L. J. Funct. Foods 2013, 5, 1732–1740. [Google Scholar] [CrossRef]
- Wang, Z.; Hwang, S.H.; Guillen Quispe, Y.N.; Gonzales Arce, P.H.; Lim, S.S. Investigation of the antioxidant and aldose reductase inhibitory activities of extracts from Peruvian tea plant infusions. Food Chem. 2017, 231, 222–230. [Google Scholar] [CrossRef]
- Kainama, H.; Fatmawati, S.; Santoso, M.; Papilaya, P.M.; Ersam, T. The relationship of free radical scavenging and total phenolic and flavonoid contents of Garcinia lasoar pam. Pharm. Chem. J. 2020, 53, 1151–1157. [Google Scholar] [CrossRef]
- Kwon, S.H.; Wang, Z.; Hwang, S.H.; Kang, Y.H.; Lee, J.Y.; Lim, S.S. Comprehensive evaluation of the antioxidant capacity of Perilla frutescens leaves extract and isolation of free radical scavengers using step-wise HSCCC guided by DPPH-HPLC. Int. J. Food Prop. 2017, 20, S921–S934. [Google Scholar] [CrossRef] [Green Version]
- Hazra, B.; Biswas, S.; Mandal, N. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement. Altern. Med. 2008, 8, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Guillen Quispe, Y.N.; Hwang, S.H.; Zuo, G.; Lim, S.S. Pistafolin B is the major aldose reductase inhibitor of the pods of tara [Caesalpinia spinose (Molina) Kuntze]. Ind. Crops Prod. 2018, 122, 709–715. [Google Scholar] [CrossRef]
- Yilmazer-Musa, M.; Griffith, A.M.; Michels, A.J.; Schneider, E.; Frei, B. Grape seed and tea extracts and catechin 3-gallates are potent inhibitors of α-amylase and α-glucosidase activity. J. Agr. Food Chem. 2012, 60, 8924–8929. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.J.; Hsieh, W.T.; Chang, H.Y.; Huang, S.S.; Lin, Y.C.; Kuo, Y.H. α-Glucosidase and aldose reductase inhibitory activities from the fruiting body of Phellinus merrillii. J. Agr. Food Chem. 2011, 59, 5702–5706. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, S.; Cui, Z.; Nie, H.; Han, D.; Yan, H. Screening and isolating major aldose reductase inhibitors from the seeds of evening primrose (Oenothera biennis). Molecules 2019, 24, 2709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalam, S.; Gul, M.Z.; Singh, R.; Ankati, S. Free radicals: Implications in etiology of chronic diseases and their amelioration through nutraceuticals. Pharmacologia 2015, 6, 11–20. [Google Scholar]
- Johanses, J.S.; Harris, A.K.; Rychly, D.J.; Ergul, A. Oxidative stress and the use of antioxidants in diabetes: Linking basic science to clinical practice. Cardiovasc. Diabetol. 2005, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Iglesias-Carres, L.; Mas-Capdevila, A.; Bravo, F.I.; Bladé, C.; Arola-Arnal, A.; Muguerza, B. Optimization of extraction methods for characterization of phenolic compounds in apricot fruit (Prunus armeniaca). Food Funct. 2019, 10, 6492–6502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutri. 2003, 78, 517S–520S. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, M.J.; Kim, D.W.; Kim, G.Y.; Kim, J.K.; Gebru, Y.A.; Choi, H.S.; Kim, Y.H.; Kim, M.K. Changes of phytochemical components (urushiols, polyphenols, gallotannins) and antioxidant capacity during fomitella fraxinea–mediated fermentation of toxicodendron vernicifluum bark. Molecules 2019, 24, 683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muccilli, V.; Cardullo, N.; Spatafora, C.; Cunsolo, V.; Tringali, C. α-Glucosidase inhibition and antioxidant activity of an oenological commercial tannin. Extraction, fractionation and analysis by HPLC/ESI-MS/MS and 1H NMR. Food Chem. 2017, 215, 50–60. [Google Scholar] [CrossRef]
- Xue, N.; Jia, Y.; Li, C.; He, B.; Yang, C.; Wang, J. Characterizations and assays of α-glucosidase inhibition activity on gallic acid cocrystals: Can the cocrystals be defined as a new chemical entity during binding with the α-glucosidase? Molecules 2020, 25, 1163. [Google Scholar] [CrossRef] [Green Version]
- Mahindrakar, K.V.; Rathod, V.K. Ultrasonic assisted aqueous extraction of catechin and gallic acid from Syzygium cumini seed kernel and evaluation of total phenolic, flavonoid contents and antioxidant activity. Chem. Eng. Process. 2020, 149, 107841. [Google Scholar] [CrossRef]
- Toshima, A.; Matsui, T.; Noguchi, M.; Qiu, J.; Tamaya, K.; Miyata, Y.; Tanaka, T.; Tanaka, K. Identification of alpha-glucosidase inhibitors from a new fermented tea obtained by tea-rolling processing of loquat (Eriobotrya japonica) and green tea leaves. J. Sci. Food Agric. 2010, 90, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jang, D.S.; Kim, N.H.; Lee, Y.M.; Kim, J.; Kim, J.S. Galloyl glucoses from the seeds of Cornus officinalis with inhibitory activity against protein glycation, aldose reductase, and cataractogenesis ex vivo. Biol. Pharm. Bull. 2011, 34, 443–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ononamadu, C.; Ihegboro, G.O.; Owolarafe, T.A.; Salawu, K.; Fadilu, M.; Ezeigwe, O.C.; Oshobu, M.L.; Nwachukwu, F.C. Identification of potential antioxidant and hypoglycemic compounds in aqueous-methanol fraction of methanolic extract of Ocimum canum leaves. Anal. Bioanal. Chem. Res. 2019, 6, 431–439. [Google Scholar]
- Nguyen, M.T.T.; Nguyen, N.T.; Nguyen, H.X.; Huynh, T.N.N.; Min, B.S. Screening of α-glucosidase inhibitory activity of vietnamese medicinal plants: Isolation of active principles from Oroxylum indicum. Nat. Prod. Sci. 2012, 18, 47–51. [Google Scholar]
- Zuo, G.L.; Kim, H.Y.; Guillen Quispe, Y.N.; Wang, Z.Q.; Hwang, S.H.; Shin, K.O.; Lim, S.S. Efficient separation of phytochemicals from Muehlenbeckia volcanica (Benth.) Endl. by polarity-stepwise elution counter-current chromatography and their antioxidant, antiglycation, and aldose reductase inhibition potentials. Molecules 2021, 26, 224. [Google Scholar] [CrossRef] [PubMed]
- Al Olayan, E.M.; Aloufi, A.S.; Alamri, O.D.; El-Habit, O.H.; Abdel Moneim, A.E. Protocatechuic acid mitigates cadmium-induced neurotoxicity in rats: Role of oxidative stress, inflammation and apoptosis. Sci. Total Environ. 2020, 723, 137969. [Google Scholar] [CrossRef]
- Zhao, L.; Wen, L.; Lu, Q.; Liu, R. Interaction mechanism between α-glucosidase and A-type trimer procyanidin revealed by integrated spectroscopic analysis techniques. Int. J. Biol. Macromol. 2020, 143, 173–180. [Google Scholar] [CrossRef]
- Martins, G.R.; do Amaral, F.R.L.; Brum, F.L.; Mohana-Borges, R.; de Moura, S.S.T.; Ferreira, F.A.; Sangenito, L.S.; Santos, A.L.S.; Figueiredo, N.G.; da Silva, A.S.A. Chemical characterization, antioxidant and antimicrobial activities of açaí seed (Euterpe oleracea Mart.) extracts containing A- and B-type procyanidins. LWT—Food Sci. Technol. 2020, 132, 109830. [Google Scholar] [CrossRef]
- Ado, M.A.; Abas, F.; Ismail, I.S.; Ghazali, H.M.; Shaari, K. Chemical profile and antiacetylcholinesterase, antityrosinase, antioxidant and α-glucosidase inhibitory activity of Cynometra cauliflora L. leaves. J. Sci. Food Agric. 2015, 95, 635–642. [Google Scholar] [CrossRef]
- Wang, W.; Bostic, T.R.; Gu, L. Antioxidant capacities, procyanidins and pigments in avocados of different strains and cultivars. Food Chem. 2010, 122, 1193–1198. [Google Scholar] [CrossRef]
- Kim, T.; Choi, H.J.; Eom, S.-H.; Lee, J.; Kim, T.H. Potential α-glucosidase inhibitors from thermal transformation of (+)-catechin. Bioorg. Med. Chem. Lett. 2014, 24, 1621–1624. [Google Scholar] [CrossRef]
- Jung, H.A.; Jung, Y.J.; Yoon, N.Y.; Jeong, D.M.; Bae, H.J.; Kim, D.-W.; Na, D.H.; Choi, J.S. Inhibitory effects of Nelumbo nucifera leaves on rat lens aldose reductase, advanced glycation endproducts formation, and oxidative stress. Food Chem. Toxicol. 2008, 46, 3818–3826. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.M.; Farhoosh, R.; Sharif, A.; Rezaie, M. Structure-antioxidant activity relationships of luteolin and catechin. J. Food Sci. 2020, 85, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Bu, Y.G.; Zhao, M.L.; Tao, R.; Luo, J.; Li, Y. Studies on antioxidant and α-glucosidase inhibitory constituents of Chinese toon bud (Toona sinensis). J. Funct. Foods 2020, 73, 104108. [Google Scholar] [CrossRef]
- Asnaashari, M.; Farhoosh, R.; Sharif, A. Antioxidant activity of gallic acid and methyl gallate in triacylglycerols of Kilka fish oil and its oil-in-water emulsion. Food Chem. 2014, 159, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Kim, S.; Yu, J.S.; Park, D.H.; Kim, S.Y.; Kang, K.S.; Lee, S.; Kim, K.H. Procyanidin B2 3”-O-gallate isolated from reynoutria elliptica prevents glutamate-induced HT22 cell death by blocking the accumulation of intracellular reactive oxygen species. Biomolecules 2019, 9, 412. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.-H.; Guo, Y.-W. Two new ellagic acid glycosides from leaves of Diplopanax stachyanthus. J. Asian Nat. Prod. Res. 2004, 6, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Talcott, S.T. Fruit maturity and juice extraction influences ellagic acid derivatives and other antioxidant polyphenolics in muscadine grapes. J. Agric. Food Chem. 2004, 52, 361–366. [Google Scholar] [CrossRef]
- Grewal, A.S.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S. Natural compounds as source of aldose reductase (AR) inhibitors for the treatment of diabetic complications: A Mini Review. Curr. Drug Metab. 2020, 21, 1091–1116. [Google Scholar] [CrossRef]
- Xu, J.Z.; Yeung, S.Y.V.; Chang, Q.; Huang, Y.; Chen, Z.-Y. Comparison of antioxidant activity and bioavailability of tea epicatechins with their epimers. Br. J. Nutr. 2004, 91, 873–881. [Google Scholar]
- Pallauf, K.; Rivas-Gonzalo, J.C.; del Castillo, M.D.; Cano, M.P.; de Pascual-Teresa, S. Characterization of the antioxidant composition of strawberry tree (Arbutus unedo L.) fruits. J. Food Compos. Anal. 2008, 21, 273–281. [Google Scholar] [CrossRef] [Green Version]
- Benalla, W.; Bellahcen, S.; Bnouham, M. Antidiabetic medicinal plants as a source of alpha glucosidase inhibitors. Curr. Diabetes Rev. 2010, 6, 247–254. [Google Scholar] [CrossRef]
- Wei, X.; Chen, D.; Yi, Y.; Qi, H.; Gao, X.; Fang, H.; Gu, Q.; Wang, L.; Gu, L. Syringic acid extracted from Herba dendrobii prevents diabetic cataract pathogenesis by inhibiting aldose reductase activity. Evid. Based Complement. Alternat. Med. 2012, 2012, 426537. [Google Scholar] [CrossRef] [Green Version]
- Vo, Q.V.; Van Bay, M.; Nam, P.C.; Quang, D.T.; Flavel, M.; Hoa, N.T.; Mechler, A. Theoretical and experimental studies of the antioxidant and antinitrosant activity of syringic acid. J. Org. Chem. 2020, 85, 15514–15520. [Google Scholar] [CrossRef]
- Miao, J.; Li, X.; Zhao, C.; Gao, X.; Wang, Y.; Gao, W. Active compounds, antioxidant activity and α-glucosidase inhibitory activity of different varieties of Chaenomeles fruits. Food Chem. 2018, 248, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Akileshwari, C.; Raghu, G.; Muthenna, P.; Mueller, N.H.; Suryanaryana, P.; Petrash, J.M.; Reddy, G.B. Bioflavonoid ellagic acid inhibits aldose reductase: Implications for prevention of diabetic complications. J. Funct. Foods 2014, 6, 374–383. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Zuccari, G. Oxidative stress, antioxidant capabilities, and bioavailability: Ellagic acid or urolithins? Antioxidants 2020, 9, 707. [Google Scholar] [CrossRef]
- Jiang, P.; Xiong, J.; Wang, F.; Grace, M.H.; Lila, M.A.; Xu, R. α-Amylase and α-glucosidase inhibitory activities of phenolic extracts from Eucalyptus grandis × E. urophylla Bark. J. Chem. 2017, 2017, 8516964. [Google Scholar] [CrossRef]
- El-Zaeddi, H.; Calín-Sánchez, Á.; Nowicka, P.; Martínez-Tomé, J.; Noguera-Artiaga, L.; Burló, F.; Wojdyło, A.; Carbonell-Barrachina, Á.A. Preharvest treatments with malic, oxalic, and acetylsalicylic acids affect the phenolic composition and antioxidant capacity of coriander, dill and parsley. Food Chem. 2017, 226, 179–186. [Google Scholar] [CrossRef]
- Rana, S.; Prakash, V.; Sagar, A. Medicinal and antioxidant properties of some medicinal plants. J. Drug Deliv. Ther. 2016, 6, 1–6. [Google Scholar] [CrossRef]
- Harb, J.; Alseekh, S.; Tohge, T.; Fernie, A.R. Profiling of primary metabolites and flavonols in leaves of two table grape varieties collected from semiarid and temperate regions. Phytochemistry 2015, 117, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Tabopda, T.K.; Ngoupayo, J.; Liu, J.; Ali, M.S.; Khan, S.N.; Ngadjui, B.T.; Luu, B. Alpha-glucosidase inhibitors ellagic acid derivatives with immunoinhibitory properties from Terminalia superba. Chem. Pharm. Bull. 2008, 56, 847–850. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Xu, Y.; Ge, Z.; Zhu, W.; Xu, Z.; Li, C. Structural elucidation and antioxidant activity evaluation of key phenolic compounds isolated from longan (Dimocarpus longan Lour.) seeds. J. Funct. Foods 2015, 17, 872–880. [Google Scholar] [CrossRef]
- Siebert, D.A.; Campos, J.S.; Alberton, M.D.; Vitali, L.; Micke, G.A. Dual electrophoretically-mediated microanalysis in multiple injection mode for the simultaneous determination of acetylcholinesterase and α-glucosidase activity applied to selected polyphenols. Talanta 2021, 224, 121773. [Google Scholar] [CrossRef]
- Ulusoy, H.G.; Sanlier, N. A minireview of quercetin: From its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. 2020, 60, 3290–3303. [Google Scholar] [CrossRef]
- Song, X.; Wang, Y.; Gao, L. Mechanism of antioxidant properties of quercetin and quercetin-DNA complex. J. Mol. Model. 2020, 26, 133. [Google Scholar] [CrossRef]



| Sample | IC50 (μg/mL) 1 | |
|---|---|---|
| α-Glucosidase | Rat Lens Aldose Reductase | |
| DSOB | 3.31 ± 0.09 a 2 | 2.56 ± 0.06 a |
| SOB | 3.18 ± 0.13 a | 3.44 ± 0.39 b |
| Positive control 3 | 1265.7 ± 29.4 b | 0.031 ± 0.001 c |
| Samples | IC50 1 (μg/mL) | ||||
|---|---|---|---|---|---|
| DPPH 2 | ABTS 3 | HO• 4 | NO 5 | ONOO− 6 | |
| DSOB | 15.62 ± 4.79 a 7 | 1.12 ± 0.11 a | Na 8 | 1.08 ± 0.20 a | 512.49 ± 9.64 a |
| SOB | 18.04 ± 5.20 ab | 1.43 ± 0.30 a | 1784.01 ± 475.14 a | 1.01 ± 0.22 a | 470.54 ± 49.68 b |
| Positive control | 33.12 ± 11.93 b | 1.34 ± 0.07 a | 110.06 ± 11.38 b | 0.039 ± 0.019 b | 94.99 ± 0.23 c |
| DSOB | SOB | p-Value 4 | |
|---|---|---|---|
| TPC (mg GAE/g dw) 1 | 48.91 ± 2.08 3 | 33.10 ± 2.27 | <0.001 |
| TFC (mg CE/g dw) 2 | 33.93 ± 0.60 | 21.97 ± 0.56 | <0.001 |
| tR (min) 1 | [M+H]+ m/z | [M-H]− m/z | Predicted Formula | Observed (m/z) | Theroetical (m/z) | Mass Error (mDa) | DBE 2 | Temporarily Identified | Activity | Content Ratio (DSOB/SOB) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.70 | - 3 | 377.0824 | C18H18O9 | 377.0824 | 377.0873 | −4.9 | 10.5 | Syringic anhydride | - | 4.47 |
| 2 | 0.72 | 381.0837 | - | C17H16O10 | 381.0837 | 381.0822 | 1.5 | 9.5 | Trimethylenglykol digalloat | - | 3.05 |
| 3 | 0.93 | - | 331.0713 | C13H16O10 | 331.0713 | 331.0665 | 4.8 | 6.5 | Monogalloylglucose | α-Glucosidase inhibitor [29]; antioxidant [30] | 1.06 |
| 4 | 1.13 | - | 331.0634 | C13H16O10 | 331.0634 | 331.0665 | −3.1 | 6.5 | Monogalloylglucose | α-Glucosidase inhibitor [29]; antioxidant [30] | 3.38 |
| 5 | 1.40 | 171.0356 | 169.0145 | C7H6O5 | 169.0145 | 169.0137 | 0.8 | 5.5 | Gallic acid | α-Glucosidase inhibitor [31]; aldose reductase inhibitor [24]; antioxidant [32] | 1.04 |
| 6 | 1.71 | 449.0734 | - | C20H16O12 | 449.0734 | 449.0720 | 1.4 | 12.5 | Methyl ellagic acid xyloside | - | 1.01 |
| 7 | 1.98 | - | 483.0811 | C20H20O14 | 483.0811 | 483.0775 | 3.6 | 11.5 | Digalloylglucose | α-Glucosidase inhibitor [33]; aldose reductase inhibitor [34]; antioxidant [35] | 0.88 |
| 8 | 2.07 | - | 153.0180 | C7H6O4 | 153.0180 | 153.0188 | −0.8 | 5.5 | Protocatechuic acid | α-Glucosidase inhibitor [36]; aldose reductase inhibitor [37]; antioxidant [38] | 1.03 |
| 9 | 2.29 | 579.1470 | 577.1298 | C30H26O12 | 579.1470 | 579.1503 | −3.3 | 17.5 | Procyanidin B | α-Glucosidase inhibitor [39]; aldose reductase inhibitor [24]; antioxidant [40] | 0.87 |
| 10 | 2.44 | 579.1470 | 577.1403 | C30H26O12 | 579.1470 | 579.1503 | −3.3 | 17.5 | Procyanidin B | α-Glucosidase inhibitor [39]; aldose reductase inhibitor [24]; antioxidant [40] | 0.71 |
| 11 | 2.52 | 867.2014 | 865.1972 | C45H38H18 | 865.1972 | 865.1980 | −0.8 | 27.5 | Procyanidin trimer | α-Glucosidase inhibitor [41]; antioxidant [42] | 0.60 |
| 12 | 2.62 | 291.0899 | 289.0694 | C15H14O6 | 289.0694 | 289.0712 | −1.8 | 9.5 | (+)-Catechin | α-Glucosidase inhibitor [43]; aldose reductase inhibitor [44]; antioxidant [45] | 0.86 |
| 13 | 2.72 | 185.0494 | 183.0301 | C8H8O5 | 183.0301 | 183.0293 | 0.8 | 5.5 | Methyl gallate | α-Glucosidase inhibitor [46]; aldose reductase inhibitor [24]; antioxidant [47] | 0.92 |
| 14 | 2.90 | 731.1622 | 729.1451 | C37H30O16 | 729.1451 | 729.1456 | −0.5 | 23.5 | Procyanidin B gallate | Antioxidant [48] | 0.82 |
| 15 | 3.04 | 465.0714 | 463.0554 | C20H16O13 | 463.0554 | 463.0513 | 4.1 | 13.5 | Ellagic acid glycoside | Aldose reductase inhibitor [49]; antioxidant [50] | 1.67 |
| 16 | 3.39 | 443.0980 | 441.0837 | C22H18O10 | 443.0980 | 443.0978 | 0.2 | 13.5 | Catechin gallate | Aldose reductase inhibitor [51]; antioxidant [52] | 0.79 |
| 17 | 3.63 | 435.0565 | 433.0388 | C19H14O12 | 435.0565 | 435.0564 | 0.1 | 12.5 | Ellagic acid xyloside | Antioxidant [53] | 1.37 |
| 18 | 3.79 | 199.0663 | 197.0456 | C9H10O5 | 197.0456 | 197.0450 | 0.6 | 5.5 | Syringic acid | α-Glucosidase inhibitor [54]; aldose reductase inhibitor [55]; antioxidant [56] | 1.87 |
| 19 | 4.09 | 303.0162 | 300.9980 | C14H6O8 | 300.9980 | 300.9984 | −0.4 | 12.5 | Ellagic acid | α-Glucosidase inhibitor [57]; aldose reductase inhibitor [58]; antioxidant [59] | 1.13 |
| 20 | 4.23 | 479.0820 | 477.0708 | C21H18O13 | 479.0820 | 479.0826 | −0.6 | 12.5 | Quercetin glucuronide | α-Glucosidase inhibitor [60]; antioxidant [61] | 1.18 |
| 21 | 4.67 | 441.0876 | 439.0679 | C22H16O10 | 439.0679 | 439.0665 | 1.4 | 15.5 | Galloylxy trihydroxyflavanone | - | 0.05 |
| 22 | 4.79 | - | 433.0752 | C20H18O11 | 433.0752 | 433.0771 | −1.9 | 12.5 | Quercetin xylopyranoside | Antioxidant [62] | 1.15 |
| 23 | 4.92 | 463.0877 | 461.0743 | C21H18O12 | 463.0877 | 463.0877 | −0.8 | 12.5 | Kaempferol glucuronide | Antioxidant [63] | 2.37 |
| 24 | 5.54 | - | 315.0179 | C15H8O8 | 315.0179 | 315.0141 | 3.8 | 12.5 | Methyl ellagic acid | α-Glucosidase inhibitor [64]; aldose reductase inhibitor [2]; antioxidant [65] | 0.88 |
| 25 | 6.99 | 303.0542 | 301.0359 | C15H10O7 | 301.0359 | 301.0348 | 1.1 | 11.5 | Quercetin | α-Glucosidase inhibitor [66]; aldose reductase inhibitor [67]; antioxidant [68] | 1.08 |
| Samples | Carbohydrates (g/100 g dw 1) | Proteins (g/100 g dw) | Ash (g/100 g dw) | Fat (g/100 g dw) | Energy (Kcal/100 g dw) |
|---|---|---|---|---|---|
| DSOB | 72.60 ± 1.12 2 | 15.86 ± 0.95 | 8.86 ± 0.24 | 2.68 ± 0.89 | 378.00 ± 4.74 |
| SOB | 56.34 ± 1.96 | 12.80 ± 0.77 | 7.00 ± 0.03 | 23.86 ± 1.16 | 491.33 ± 5.68 |
| p-Value 3 | 0.002 | 0.013 | 0.001 | <0.001 | <0.001 |
| Sample | DSOB | SOB | p-Value 1 |
|---|---|---|---|
| Glucose (g/100 g dw 2) | 0.16 ± 0.010 3 | 0.13 ± 0.006 | 0.034 |
| Fructose (g/100 g dw) | ND 4 | ND | - 5 |
| Galactose (g/100 g dw) | ND | ND | - |
| Sucrose (g/100 g dw) | 1.27 ± 0.08 | 0.99 ± 0.04 | 0.042 |
| Maltose (g/100 g dw) | ND | 0.13 ± 0.005 | <0.001 |
| Lactose (g/100 g dw) | ND | ND | - |
| Fibers (g/100 g dw) | 34.78 ± 2.50 | 29.40 ± 1.28 | 0.03 |
| Amino Acids | Amino Acid (g/100 g Protein) | p-Value 2 | CS 3 | p-Value | |||
|---|---|---|---|---|---|---|---|
| DSOB | SOB | DSOB | SOB | ||||
| Essential amino acid | |||||||
| Valine | 3.12 ± 0.19 1 | 3.28 ± 0.20 | 0.493 | 47.27 ± 2.84 | 49.70 ± 2.98 | 0.487 | |
| Isoleucine | 1.68 ± 0.10 | 1.72 ± 0.10 | 0.730 | 31.11 ± 1.87 | 31.85 ± 1.91 | 0.735 | |
| Leucine | 4.40 ± 0.26 | 4.69 ± 0.28 | 0.384 | 51.16 ± 3.07 | 54.53 ± 3.27 | 0.388 | |
| Threonine | 2.32 ± 0.14 | 2.50 ± 0.15 | 0.324 | 49.36 ± 2.96 | 53.19 ± 3.19 | 0.323 | |
| Lysine | 2.32 ± 0.14 | 2.60 ± 0.16 | 0.175 | 33.14 ± 1.99 | 37.14 ± 2.23 | 0.170 | |
| Histidine | 1.28 ± 0.08 | 1.48 ± 0.09 | 0.112 | 58.18 ± 3.49 | 67.27 ± 4.04 | 0.106 | |
| Tyrosine | 1.52 ± 0.09 | 1.49 ± 0.09 | 0.773 | 54.19 ± 3.25 | 55.48 ± 3.33 | 0.736 | |
| Phenylalanine | 3.52 ± 0.21 | 3.67 ± 0.22 | 0.555 | ||||
| Cysteine | 1.52 ± 0.09 | 1.47 ± 0.09 | 0.635 | 42.11 ± 2.53 | 44.91 ± 2.69 | 0.383 | |
| Methionine | 0.88 ± 0.05 | 1.09 ± 0.07 | 0.047 | ||||
| Non-essential amino acid | |||||||
| Arginine | 6.72 ± 0.40 | 7.27 ± 0.44 | 0.303 | ||||
| Proline | 5.76 ± 0.35 | 6.71 ± 0.40 | 0.096 | ||||
| Aspartic | 6.16 ± 0.37 | 6.72 ± 0.40 | 0.262 | ||||
| Serine | 4.32 ± 0.26 | 4.61 ± 0.28 | 0.384 | ||||
| Glutamic acid | 14.24 ± 0.85 | 15.63 ± 0.94 | 0.238 | ||||
| Glycine | 4.88 ± 0.29 | 5.23 ± 0.31 | 0.349 | ||||
| Alanine | 2.72 ± 0.16 | 2.81 ± 0.17 | 0.641 | ||||
| EAAI 4 | 44.84 ± 2.69 | 48.09 ± 2.89 | 0.350 | ||||
| BV 5 | 37.15 ± 2.93 | 40.68 ± 3.14 | 0.350 | ||||
| Sample | DSOB | SOB | p-Value 3 |
|---|---|---|---|
| Macroelements (mg/100 g dw 1) | |||
| Na | 1121.67 ± 3.45 2 | 999.44 ± 6.20 | <0.001 |
| K | 259.63 ± 1.50 | 303.67 ± 0.87 | <0.001 |
| Ca | 1106.77 ± 7.73 | 1001.69 ± 7.08 | <0.001 |
| Mg | 320.88 ± 1.34 | 243.74 ± 1.01 | <0.001 |
| Microelements (mg/100 g dw) | |||
| Fe | 50.35 ± 0.63 | 42.86 ± 0.25 | <0.001 |
| Mn | 7.22 ± 0.04 | 5.72 ± 0.12 | <0.001 |
| Cu | 0.40 ± 0.003 | 0.43 ± 0.008 | 0.016 |
| Zn | 3.60 ± 0.001 | 2.02 ± 0.05 | <0.001 |
| Sample | DSOB | SOB | p-Value 3 |
|---|---|---|---|
| Oxalic acid (mg/100 g dw 1) | 255.30 ± 30.00 2 | 584.65 ± 52.23 | 0.002 |
| Citric acid (mg/100 g dw) | 363.26 ± 18.05 | 171.67 ± 22.67 | 0.001 |
| α-Tocopherol (mg/100 g dw) | ND 4 | ND | - 5 |
| β-Tocopherol (mg/100 g dw) | ND | ND | - |
| γ-Tocopherol (mg/100 g dw) | 1.11 ± 0.001 | 11.61 ± 0.04 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wu, Z.; Zuo, G.; Lim, S.S.; Yan, H. Defatted Seeds of Oenothera biennis as a Potential Functional Food Ingredient for Diabetes. Foods 2021, 10, 538. https://doi.org/10.3390/foods10030538
Wang Z, Wu Z, Zuo G, Lim SS, Yan H. Defatted Seeds of Oenothera biennis as a Potential Functional Food Ingredient for Diabetes. Foods. 2021; 10(3):538. https://doi.org/10.3390/foods10030538
Chicago/Turabian StyleWang, Zhiqiang, Zhaoyang Wu, Guanglei Zuo, Soon Sung Lim, and Hongyuan Yan. 2021. "Defatted Seeds of Oenothera biennis as a Potential Functional Food Ingredient for Diabetes" Foods 10, no. 3: 538. https://doi.org/10.3390/foods10030538
APA StyleWang, Z., Wu, Z., Zuo, G., Lim, S. S., & Yan, H. (2021). Defatted Seeds of Oenothera biennis as a Potential Functional Food Ingredient for Diabetes. Foods, 10(3), 538. https://doi.org/10.3390/foods10030538