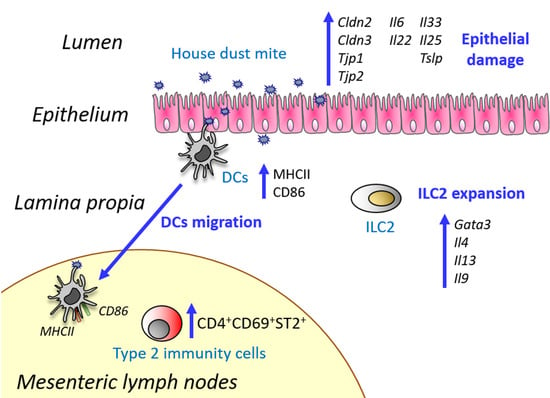
Oral Exposure to House Dust Mite Activates Intestinal Innate Immunity
Abstract
:1. Introduction
2. Experimental Section
2.1. Inactivation of The House Dust Mite Extract
2.2. Protease Activity of the House Dust Mite Extract
2.3. Hydrolysis of Ovalbumin by House Dust Mite
2.4. Experiments in Mice
2.5. Isolation of Lamina Propria Cells
2.6. Bone Marrow Derived Dendritic Cells
2.7. Gene Expression Analyses
2.8. Flow Cytometry Analyses
2.9. Statistical Analyses
3. Results
3.1. Oral Exposure to Proteolytically Active House Dust Mite Changes the Expression of Genes Related to Intestinal Epithelial Barrier Integrity
3.2. House Dust Mite Increases Intestinal Th2 Responses
3.3. House Dust Mite causes Dendritic Cell Activation In Vitro Irrespectively of Its Proteolytic Activity
3.4. House Dust Mite Does Not Promote Sensitization to Coadministered Food Allergens
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tordesillas, L.; Berin, M.V.; Sampson, H.A. Immunology of food allergy. Immunity 2017, 47, 32–50. [Google Scholar] [CrossRef] [Green Version]
- Cayrol, C.; Duval, A.; Schmitt, P.; Roga, S.; Camus, M.; Stella, A.; Burlet-Schiltz, O.; Gonzalez-de-Peredo, A.; Girard, J.P. Environmental allergens induce allergic inflammation through proteolytic maturation of IL-33. Nat. Immunol. 2018, 19, 375–385. [Google Scholar] [CrossRef]
- Jacquet, A. Innate immune responses in house dust mite allergy. ISRN Allergy 2013, 2013, 735031. [Google Scholar] [CrossRef] [Green Version]
- Reithofer, M.; Jahn-Schmid, B. Allergens with protease activity from house dust mites. Int. J. Mol. Sci. 2017, 18, 1368. [Google Scholar] [CrossRef] [Green Version]
- Sokol, C.L.; Barton, G.M.; Farr, A.G.; Medzhitov, R.A. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat. Immunol. 2008, 9, 310–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Zeng, X.; He, S. Evaluation on potential contributions of protease activated receptors related mediators in allergic inflammation. Mediators Inflamm. 2014, 2014, 829068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Post, S.; Nawijn, M.C.; Hackett, T.L.; Baranowska, M.; Gras, R.; van Oosterhout, A.J.M.; Heijink, I.H. The composition of house dust mite is critical for mucosal barrier dysfunction and allergic sensitization. Thorax 2012, 67, 488–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papazian, D.; Hansen, S.; Wurtzen, P.A. Airway responses towards allergens—From the airway epithelium to T cells. Clin. Exp. Allergy 2015, 45, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Divanovic, S.; Visintin, A.; Blanchard, C.; Hegde, R.S.; Madan, R.; Thorne, P.S.; Wills-Karp, M.; Gioannini, T.L.; Weiss, J.P.; et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature 2009, 457, 585–588. [Google Scholar] [CrossRef]
- Choi, J.P.; Lee, S.M.; Choi, H.I.; Kim, M.H.; Jeon, S.G.; Jang, M.H.; Jee, Y.J.; Yang, S.; Cho, Y.J.; Kim, Y.K. House dust mite-derived chitin enhances th2 cell response to inhaled allergens, mainly via a tnf-α-dependent pathway. Allergy Asthma Immunol. Res. 2016, 8, 362–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tulic, M.K.; Vivinus-Nebot, M.; Rekima, A.; Rabelo Medeiros, S.; Bonnart, C.; Shi, H.; Walker, A.; Dainese, R.; Boyer, J.; Vergnolle, N.; et al. Presence of commensal house dust mite allergen in human gastrointestinal tract: A potential contributor to intestinal barrier dysfunction. Gut 2016, 65, 757–766. [Google Scholar] [CrossRef]
- Macchiaverni, P.; Rekima, A.; Turfkruyer, M.; Mascarell, L.; Airouche, S.; Moingeon, P.; Adel-Patient, K.; Condino-Neto, A.; Annesi-Maesano, I.; Prescott, S.L.; et al. Respiratory allergen from house dust mite is present in human milk and primes for allergic sensitization in a mouse model of asthma. Allergy 2014, 69, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D. The role of dust mites in allergy. Clin. Rev. Allergy Immunol. 2019, 57, 312–329. [Google Scholar] [CrossRef] [PubMed]
- Samadi, N.; Klems, M.; Untersmayr, E. The role of gastrointestinal permeability in food allergy. Ann. Allergy Asthma Immunol. 2018, 121, P168–P173. [Google Scholar] [CrossRef] [PubMed]
- Roan, F.; Obata-Ninomiya, K.; Ziegler, S.F. Epithelial cell—Derived cytokines: More than just signaling the alarm. J. Clin. Investig. 2019, 129, 1441–1451. [Google Scholar] [CrossRef] [Green Version]
- Pablos-Tanarro, A.; Lozano-Ojalvo, D.; Martínez-Blanco, M.; Molina, E.; López-Fandiño, R. Egg yolk provides th2 adjuvant stimuli and promotes sensitization to egg white allergens in BALB/c mice. Mol. Nutr. Food Res. 2018, 62, e1800057. [Google Scholar] [CrossRef] [PubMed]
- Schulz, O.; Sewell, H.F.; Shakib, F.A. A sensitive fluorescent assay for measuring the cysteine protease activity of Der p 1, a major allergen from the dust mite Dermatophagoides pteronyssinus. Mol. Pathol. 1998, 51, 222–224. [Google Scholar] [CrossRef] [Green Version]
- Benedé, S.; López-Fandiño, R.; Reche, M.; Molina, E.; López-Expósito, I. Influence of the carbohydrate moieties on the immunoreactivity and digestibility of the egg allergen ovomucoid. PLoS ONE 2013, 8, e80810. [Google Scholar]
- Wang, Y.T.; Liu, H.C.; Chen, H.C.; Lee, Y.C.; Tsai, T.C.; Chen, H.L.; Fan, H.C.; Chen, C.M. Oral immunotherapy with the ingestion of house dust mite extract in a murine model of allergic asthma. Allergy Asthma Clin. Immunol. 2018, 14, 43. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, L.; Martínez-Blanco, M.; Lozano-Ojalvo, D.; Molina, E.; López-Fandiño, R. Egg yolk augments type 2 immunity by activating innate cells. Eur. J. Nutr. 2020, 59, 3245–3256. [Google Scholar] [CrossRef]
- Volynets, V.; Rings, A.; Bárdos, G.; Ostaff, M.J.; Wehkamp, J.; Bischoffa, S.C. Intestinal barrier analysis by assessment of mucins, tight junctions, and α-defensins in healthy C57BL/6J and BALB/cJ mice. Tissue Barriers 2016, 4, e1208468. [Google Scholar] [CrossRef] [Green Version]
- Johansson, M.E.V.; Larsson, J.M.H.; Hansson, C.G. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host–microbial interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 4659–4665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zenewicz, L.A.; Flawell, R.A. Recent advances in IL-22 biology. Int. Immunol. 2011, 23, 159–163. [Google Scholar] [CrossRef] [Green Version]
- McConnell, E.L.; Basit, A.W.; Murdan, S. Measurements of rat and mouse gastrointestinal pH, fluid and lymphoid tissue, and implications for in-vivo experiments. J. Pharm. Pharmacol. 2010, 60, 63–70. [Google Scholar] [CrossRef]
- Bourlieu, C.; Ménard, O.; Bouzerzour, K.; Mandalari, G.; Macierzanka, A.; Mackie, A.R.; Dupont, D. Specificity of infant digestive conditions: Some clues for developing relevant in vitro models. Crit. Rev. Food Sci. Nutr. 2014, 54, 1427–1457. [Google Scholar] [CrossRef] [PubMed]
- Noval Rivas, M.; Burton, O.T.; Oettgen, H.C.; Chatila, T. IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. J. Allergy Clin. Immunol. 2016, 138, 801–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stier, M.T.; Peebles, R.S. Innate lymphoid cells and allergic disease. Ann. Allergy Asthma Immunol. 2017, 119, 480–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamoutounour, S.; Henri, S.; Lelouard, H.; de Bovis, B.; de Haar, C.; van der Woude, C.J.; Woltman, A.M.; Reyal, Y.; Bonnet, D.; Sichien, S.; et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur. J. Immunol. 2012, 42, 3150–3166. [Google Scholar] [CrossRef] [PubMed]
- Mildner, A.; Jung, S. development and function of dendritic cell subsets. Immunity 2014, 60, 642–656. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, S.J.; Perona-Wright, G.; Worsley, A.G.F.; Ishii, N.; MacDonald, A.S. Dendritic cell expression of OX40 ligand acts as a costimulatory, not polarizing, signal for optimal Th2 priming and memory induction in vivo. J. Immunol. 2007, 179, 3515–3523. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.W.; Tjota, M.Y.; Clay, B.S.; Vander Lugt, B.; Bandukwala, H.S.; Hrusch, C.L.; Decker, D.C.; Blaine, K.M.; Fixsen, B.R.; Singh, H.; et al. Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat. Commun. 2013, 4, 2990. [Google Scholar] [CrossRef] [Green Version]
- Amsen, D.; Helbig, C.; Backer, R.A. Notch in T cell differentiation: All things considered. Trends Immunol. 2015, 36, 802–814. [Google Scholar] [CrossRef]
- Griesenauer, B.; Paczesny, S. The ST2/IL-33 Axis in immune cells during inflammatory diseases. Front. Immunol. 2017, 8, 475. [Google Scholar] [CrossRef]
- Blazquez, A.B.; Berin, C. Gastrointestinal dendritic cells promote th2 skewing via OX40L. J. Immunol. 2008, 180, 4441–4450. [Google Scholar] [CrossRef]
- Benedé, S.; López-Expósito, I.; Molina, E.; López-Fandiño, R. Egg proteins as allergens and the effects of the food matrix and processing. Food Func. 2015, 6, 694–713. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.; Gentile, M.; Yeiser, J.R.; Walland, A.C.; Bornstein, V.U.; Chen, K.; He, B.; Cassis, L.; Bigas, A.; Cols, M.; et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 2013, 342, 447–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, C.C.; Ownby, D.R. Allergies and asthma: Do atopic disorders result from inadequate immune homeostasis arising from infant gut dysbiosis? Expert Rev. Clin. Immunol. 2016, 12, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.T.T.; Hagner, S.; Ruchti, F.; Radzikowska, U.; Tan, G.; Altunbulakli, C.; Eljaszewicz, A.; Moniuszko, M.; Akdis, M.; Akdis, C.A.; et al. Tight junction, mucin, and inflammasome-related molecules are differentially expressed in eosinophilic, mixed, and neutrophilic experimental asthma in mice. Allergy 2019, 74, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef]
- Guo, W.; Wang, P.; Liu, Z.H.; Ye, P. Analysis of differential expression of tight junction proteins in cultured oral epithelial cells altered by Porphyromonas gingivalis, Porphyromonas gingivalis lipopolysaccharide, and extracellular adenosine triphosphate. Int. J. Oral Sci. 2018, 10, e8. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Villablanca, E.J.; De Calisto, J.; Gomes, D.C.; Nguyen, D.D.; Mizoguchi, E.; Kagan, J.C.; Reinecker, H.C.; Hacohen, N.; Nagler, C.; et al. MyD88-dependent TLR1/2 signals educate dendritic cells with gut-specific imprinting properties. J. Immunol. 2011, 187, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.N.; Jiang, D.S.; Li, H. Interferon regulatory factors: At the crossroads of immunity, metabolism, and disease. Biochim. Biophys. Acta 2015, 1852, 365–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, J.H.; Yoo, J.Y.; Kim, M.J.; Hwang, S.G.; Ahn, K.C.; Ryu, J.C.; Choi, M.K.; Joo, J.H.; Kim, C.H.; Lee, S.N.; et al. Distinct TLR-mediated pathways regulate house dust mite-induced allergic disease in the upper and lower airways. J. Allergy Clin. Immunol. 2013, 131, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Chiou, Y.L.; Lin, C.Y. Der p2 activates airwaysmooth muscle cells in a TLR2/MyS88-dependent manner toinduce an inflammatory response. J. Cell Physiol. 2009, 220, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.U.; Lee, S.H.; Hwang, W.; Lee, C.; Park, J.W.; Sohn, J.H.; Nam, J.H.; Kim, Y.; Rhee, J.H.; Im, S.H.; et al. Flagellin suppresses experimental asthma by generating regulatory dendritic cells and T cells. J. Allergy Clin. Immunol. 2016, 137, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Devaney, J.M.; Greene, C.M.; Taggart, C.C.; Carroll, T.P.; O’Neill, S.J.; McElvaney, N.G. Neutrophil elastase up-regulates interleukin-8 via toll-like receptor 4. FEBS Lett. 2003, 544, 129–132. [Google Scholar] [CrossRef] [Green Version]
- Rallabhandi, P.; Nhu, Q.M.; Toshchakov, V.Y.; Piao, W.; Medvedev, A.E.; Hollenberg, M.D.; Fasano, A.; Vogel, S.N. Analysis of proteinase-activated receptor 2 and TLR4 signal transduction: A novel paradigm for receptor cooperativity. J. Biol. Chem. 2008, 283, 24314–24325. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedé, S.; Pérez-Rodríguez, L.; Martínez-Blanco, M.; Molina, E.; López-Fandiño, R. Oral Exposure to House Dust Mite Activates Intestinal Innate Immunity. Foods 2021, 10, 561. https://doi.org/10.3390/foods10030561
Benedé S, Pérez-Rodríguez L, Martínez-Blanco M, Molina E, López-Fandiño R. Oral Exposure to House Dust Mite Activates Intestinal Innate Immunity. Foods. 2021; 10(3):561. https://doi.org/10.3390/foods10030561
Chicago/Turabian StyleBenedé, Sara, Leticia Pérez-Rodríguez, Mónica Martínez-Blanco, Elena Molina, and Rosina López-Fandiño. 2021. "Oral Exposure to House Dust Mite Activates Intestinal Innate Immunity" Foods 10, no. 3: 561. https://doi.org/10.3390/foods10030561
APA StyleBenedé, S., Pérez-Rodríguez, L., Martínez-Blanco, M., Molina, E., & López-Fandiño, R. (2021). Oral Exposure to House Dust Mite Activates Intestinal Innate Immunity. Foods, 10(3), 561. https://doi.org/10.3390/foods10030561