Comparative Assessment of Tubular Ceramic, Spiral Wound, and Hollow Fiber Membrane Microfiltration Module Systems for Milk Protein Fractionation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Milk
2.2. Membrane Modules
2.3. Conditioning and Cleaning Procedure
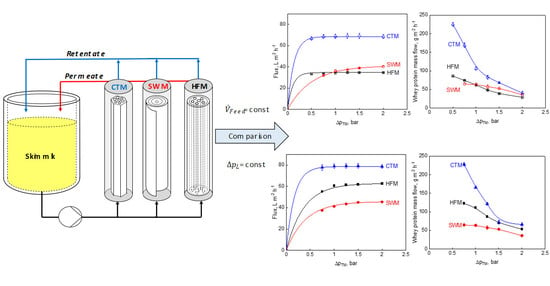
2.4. Filtration Plant
2.5. Filtration Conditions
2.6. Analysis of Caseins and Whey Proteins
2.7. Calculations
2.8. Statistics
3. Results and Discussion
3.1. Influence of the Module Configuration on the Flux
3.2. Influence of the Module Configuration on Milk Protein Transmission
3.3. Impact of the Filtration Module on the Fractionation Efficiency
3.4. Impact of the Packing Density of the Module on the Filtration Efficiency
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Samuelsson, G.; Dejmek, P.; Trägårdh, G.; Paulsson, M. Minimizing whey protein retention in cross-flow microfiltration of skim milk. Int. Dairy J. 1997, 7, 237–242. [Google Scholar] [CrossRef]
- Vadi, P.; Rizvi, S. Experimental evaluation of a uniform transmembrane pressure crossflow microfiltration unit for the concentration of micellar casein from skim milk. J. Membr. Sci. 2001, 189, 69–82. [Google Scholar] [CrossRef]
- Hernandez, A.; Harte, F. Isolation of caseins from whey proteins by microfiltration modifying the mineral balance in skim milk. J. Dairy Sci. 2009, 92, 5357–5362. [Google Scholar] [CrossRef] [PubMed]
- Le Berre, O.; Daufin, G. Skimmilk crossflow microfiltration performance versus permeation flux to wall shear stress ratio. J. Membr. Sci. 1996, 117, 261–270. [Google Scholar] [CrossRef]
- Adams, M.C.; Barbano, D.M. Effect of ceramic membrane channel diameter on limiting retentate protein concentration during skim milk microfiltration. J. Dairy Sci. 2016, 99, 167–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiffer, S.; Kulozik, U. Effect of Temperature-Dependent Bacterial Growth during Milk Protein Fractionation by Means of 0.1 µM Microfiltration on the Length of Possible Production Cycle Times. Membranes 2020, 10, 326. [Google Scholar] [CrossRef]
- Gésan-Guiziou, G.; Daufin, G.; Boyaval, E. Critical stability conditions in skimmed milk crossflow microfiltration: Impact on operating modes. Le Lait 2000, 80, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Samuelsson, G. Predicting limiting flux of skim milk in crossflow microfiltration. J. Membr. Sci. 1997, 129, 277–281. [Google Scholar] [CrossRef]
- Beckman, S.; Barbano, D. Effect of microfiltration concentration factor on serum protein removal from skim milk using spiral-wound polymeric membranes. J. Dairy Sci. 2013, 96, 6199–6212. [Google Scholar] [CrossRef]
- Zulewska, J.; Newbold, M.; Barbano, D. Efficiency of serum protein removal from skim milk with ceramic and polymeric membranes at 50 °C. J. Dairy Sci. 2009, 92, 1361–1377. [Google Scholar] [CrossRef]
- Hartinger, M.; Heidebrecht, H.-J.; Schiffer, S.; Dumpler, J.; Kulozik, U. Milk Protein Fractionation by Means of Spiral-Wound Microfiltration Membranes: Effect of the Pressure Adjustment Mode and Temperature on Flux and Protein Permeation. Foods 2019, 8, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zulewska, J.; Barbano, D.M. Influence of casein on flux and passage of serum proteins during microfiltration using polymeric spiral-wound membranes at 50 °C. J. Dairy Sci. 2013, 96, 2048–2060. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications, 2nd ed.; Wiley: Chichester, UK, 2004; ISBN 0470020393. [Google Scholar]
- von der Schulenburg, D.A.G.; Vrouwnvelder, J.S.; Creber, S.A.; van Loosdrecht, M.C.M.; Johns, M.L. Nuclear magnetic resonance microscopy studies of membrane biofouling. J. Membr. Sci. 2008, 323, 37–44. [Google Scholar] [CrossRef]
- Li, X.; Mo, Y.; Li, J.; Guo, W.; Ngo, H.H. In-situ monitoring techniques for membrane fouling and local filtration characteristics in hollow fiber membrane processes: A critical review. J. Membr. Sci. 2017, 528, 187–200. [Google Scholar] [CrossRef]
- Schork, N.; Schuhmann, S.; Arndt, F.; Schütz, S.; Guthausen, G.; Nirschl, H. MRI investigations of filtration: Fouling and cleaning processes. Microporous Mesoporous Mater. 2018, 269, 60–64. [Google Scholar] [CrossRef]
- Schopf, R.; Schork, N.; Amling, E.; Nirschl, H.; Guthausen, G.; Kulozik, U. Structural Characterisation of Deposit Layer during Milk Protein Microfiltration by Means of In-Situ MRI and Compositional Analysis. Membranes 2020, 10, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinberger, M.E.; Kulozik, U. Effect of low-frequency pulsatile crossflow microfiltration on flux and protein transmission in milk protein fractionation. Sep. Sci. Technol. 2021, 56, 1112–1127. [Google Scholar] [CrossRef]
- Schopf, R.; Schmidt, F.; Kulozik, U. Impact of hollow fiber membrane length on the milk protein fractionation. J. Membr. Sci. 2021, 620, 118834. [Google Scholar] [CrossRef]
- Bouchoux, A.; Gésan-Guiziou, G.; Pérez, J.; Cabane, B. How to Squeeze a Sponge: Casein Micelles under Osmotic Stress, a SAXS Study. Biophys. J. 2010, 99, 3754–3762. [Google Scholar] [CrossRef] [Green Version]
- Doudiès, F.; Loginov, M.; Hengl, N.; Karrouch, M.; Leconte, N.; Garnier-Lambrouin, F.; Pérez, J.; Pignon, F.; Gésan-Guiziou, G. Build-up and relaxation of membrane fouling deposits produced during crossflow ultrafiltration of casein micelle dispersions at 12 °C and 42 °C probed by in situ SAXS. J. Membr. Sci. 2021, 618, 118700. [Google Scholar] [CrossRef]
- Loginov, M.; Doudiès, F.; Hengl, N.; Pignon, F.; Gésan-Guiziou, G. Influence of membrane resistance on swelling and removal of colloidal filter cake after filtration pressure release. J. Membr. Sci. 2020, 595, 117498. [Google Scholar] [CrossRef]
- Bacchin, P.; Si-Hassen, D.; Starov, V.; Clifton, M.; Aimar, P. A unifying model for concentration polarization, gel-layer formation and particle deposition in cross-flow membrane filtration of colloidal suspensions. Chem. Eng. Sci. 2002, 57, 77–91. [Google Scholar] [CrossRef] [Green Version]
- Qu, P.; Gésan-Guiziou, G.; Bouchoux, A. Dead-end filtration of sponge-like colloids: The case of casein micelle. J. Membr. Sci. 2012, 417-418, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Bouchoux, A.; Cayemitte, P.-E.; Jardin, J.; Gésan-Guiziou, G.; Cabane, B. Casein Micelle Dispersions under Osmotic Stress. Biophys. J. 2009, 96, 693–706. [Google Scholar] [CrossRef]
- Kavianipour, O.; Ingram, G.D.; Vuthaluru, H.B. Investigation into the effectiveness of feed spacer configurations for reverse osmosis membrane modules using Computational Fluid Dynamics. J. Membr. Sci. 2017, 526, 156–171. [Google Scholar] [CrossRef] [Green Version]
- Karabelas, A.J.; Koutsou, C.P.; Sioutopoulos, D.C. Comprehensive performance assessment of spacers in spiral-wound membrane modules accounting for compressibility effects. J. Membr. Sci. 2018, 549, 602–615. [Google Scholar] [CrossRef]
- Adams, M.C.; Hurt, E.E.; Barbano, D.M. Effect of ceramic membrane channel geometry and uniform transmembrane pressure on limiting flux and serum protein removal during skim milk microfiltration. J. Dairy Sci. 2015, 98, 7527–7543. [Google Scholar] [CrossRef] [Green Version]
- Hurt, E.; Zulewska, J.; Newbold, M.; Barbano, D. Micellar casein concentrate production with a 3X, 3-stage, uniform transmembrane pressure ceramic membrane process at 50 °C. J. Dairy Sci. 2010, 93, 5588–5600. [Google Scholar] [CrossRef] [PubMed]
- Hurt, E.; Adams, M.; Barbano, D. Microfiltration of skim milk and modified skim milk using a 0.1-µm ceramic uniform transmembrane pressure system at temperatures of 50, 55, 60, and 65 °C. J. Dairy Sci. 2015, 98, 765–780. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.C.; Barbano, D.M. Serum protein removal from skim milk with a 3-stage, 3× ceramic Isoflux membrane process at 50 °C. J. Dairy Sci. 2013, 96, 2020–2034. [Google Scholar] [CrossRef]
- Dumpler, J.; Wohlschläger, H.; Kulozik, U. Dissociation and coagulation of caseins and whey proteins in concentrated skim milk heated by direct steam injection. Dairy Sci. Technol. 2016, 96, 807–826. [Google Scholar] [CrossRef]
- Gésan-Guiziou, G.; Daufin, G.; Boyaval, E.; Le Berre, O. Wall shear stress: Effective parameter for the characterisation of the cross-flow transport in turbulent regime during skimmed milk microfiltration. Le Lait 1999, 79, 347–354. [Google Scholar] [CrossRef]
- Warncke, M.; Kulozik, U. Impact of temperature and high pressure homogenization on the solubility and rheological behavior of reconstituted dairy powders of different composition. Powder Technol. 2020, 376, 285–295. [Google Scholar] [CrossRef]
- Hartinger, M.; Schiffer, S.; Heidebrecht, H.-J.; Dumpler, J.; Kulozik, U. Milk protein fractionation by custom-made prototypes of spiral-wound microfiltration membranes operated at extreme crossflow velocities. J. Membr. Sci. 2020, 605, 118110. [Google Scholar] [CrossRef]
- Field, R.; Wu, D.; Howell, J.; Gupta, B. Critical flux concept for microfiltration fouling. J. Membr. Sci. 1995, 100, 259–272. [Google Scholar] [CrossRef]
- Baruah, G.L.; Nayak, A.; Belfort, G. Scale-up from laboratory microfiltration to a ceramic pilot plant: Design and performance. J. Membr. Sci. 2006, 274, 56–63. [Google Scholar] [CrossRef]
- Schiffer, S.; Hartinger, M.; Matyssek, A.; Kulozik, U. On the reversibility of deposit formation in low temperature milk microfiltration with ceramic membranes depending on mode of adjustment of transmembrane pressure and wall shear stress. Sep. Purif. Technol. 2020, 247, 116962. [Google Scholar] [CrossRef]
- Farhat, N.; Staal, M.; Bucs, S.; Van Loosdrecht, M.; Vrouwenvelder, J. Spatial heterogeneity of biofouling under different cross-flow velocities in reverse osmosis membrane systems. J. Membr. Sci. 2016, 520, 964–971. [Google Scholar] [CrossRef]
- Hartinger, M.; Kulozik, U. Milk protein fractionation by spiral-wound microfiltration membranes in diafiltration mode—Influence of feed protein concentration and composition on the filtration performance. Int. Dairy J. 2020, 102, 104606. [Google Scholar] [CrossRef]




| Properties | 55 °C |
|---|---|
| ηWater [10−6·kg·m−1·s−1] | 504.2 ± 8.7 |
| ηMilk [10−6·kg·m−1·s−1] | 922.1 ± 11.3 |
| ηPermeate [10−6·kg·m−1·s−1] | 730.8 ± 5.9 |
| φWater [kg·m−3] | 985.7 ± 1.6 |
| φMilk [kg·m−3] | 1022.5 ± 4.7 |
| φPermeate [kg·m−3] | 1009.4 ± 2.1 |
| Type | Module Length, m | Module Diameter, m | Membrane Surface, m2 | Module Volume, L | Packing Density, m2·m−3 |
|---|---|---|---|---|---|
| HFM | 1.02 | 0.16 | 9.3 | 20.7 | 449 |
| SWM | 0.97 | 0.17 | 16.4 | 22.6 | 723 |
| CTM | 1.02 | 0.13 | 1.7 | 14.4 | 119 |
| Type | Mean Crossflow Velocity v, m·s−1 | Pressure Drop ∆pL, bar·m−1 | Wall Shear Stress τw, Pa | Wall Shear Rate γT, 103·s−1 |
|---|---|---|---|---|
| HFM | 1.6 ± 0.01 | 0.6 ± 0.01 | 23 ± 2.1 | 25 ± 2.1 |
| SWM | 0.6 ± 0.01 | 1.3 ± 0.03 | - | - |
| CTM | 3.3 ± 0.01 | 0.8 ± 0.04 | 76 ± 8.4 | 82 ± 10.2 |
| Type | Mean Crossflow Velocity v, m·s−1 | Pressure Drop ∆pL, bar·m−1 | Wall Shear Stress τw, Pa | Wall Shear Rate γT, 103·s−1 |
|---|---|---|---|---|
| HFM | 3.2 ± 0.01 | 1.3 ± 0.01 | 48 ± 2.1 | 52 ± 2.9 |
| SWM | 0.6 ± 0.01 | 1.3 ± 0.03 | - | - |
| CTM | 4.7 ± 0.01 | 1.3 ± 0.04 | 127 ± 8.4 | 138 ± 9.5 |
| Module | HFM | CTM | SWM | HFM | CTM | SWM |
|---|---|---|---|---|---|---|
| = 20 m3·h−1 | ∆pL = 1.3 bar·m−1 | |||||
| ∆pTM optimum, bar | 0.5 | 0.5 | 0.75 | 0.75 | 0.75 | 0.75 |
| Flux, L·m−2·h−1 | 33 | 67 | 32 | 55 | 78 | 32 |
| Whey protein transmission, % | 50 | 67 | 46 | 44 | 51 | 46 |
| Whey protein mass flow, g·m−2·h−1 | 86 | 225 | 64 | 123 | 229 | 64 |
| Whey protein mass flow per module, g·h−1 | 800 | 384 | 1050 | 1144 | 389 | 1050 |
| Whey protein mass flow per volume, g·m−3·h−1 | 38,614 | 26,775 | 46,272 | 55,227 | 27,251 | 46,272 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schopf, R.; Schmidt, F.; Linner, J.; Kulozik, U. Comparative Assessment of Tubular Ceramic, Spiral Wound, and Hollow Fiber Membrane Microfiltration Module Systems for Milk Protein Fractionation. Foods 2021, 10, 692. https://doi.org/10.3390/foods10040692
Schopf R, Schmidt F, Linner J, Kulozik U. Comparative Assessment of Tubular Ceramic, Spiral Wound, and Hollow Fiber Membrane Microfiltration Module Systems for Milk Protein Fractionation. Foods. 2021; 10(4):692. https://doi.org/10.3390/foods10040692
Chicago/Turabian StyleSchopf, Roland, Florian Schmidt, Johanna Linner, and Ulrich Kulozik. 2021. "Comparative Assessment of Tubular Ceramic, Spiral Wound, and Hollow Fiber Membrane Microfiltration Module Systems for Milk Protein Fractionation" Foods 10, no. 4: 692. https://doi.org/10.3390/foods10040692
APA StyleSchopf, R., Schmidt, F., Linner, J., & Kulozik, U. (2021). Comparative Assessment of Tubular Ceramic, Spiral Wound, and Hollow Fiber Membrane Microfiltration Module Systems for Milk Protein Fractionation. Foods, 10(4), 692. https://doi.org/10.3390/foods10040692