Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview
Abstract
:1. Introduction
2. Research Methods
3. Plant-Derived Bioactive Components
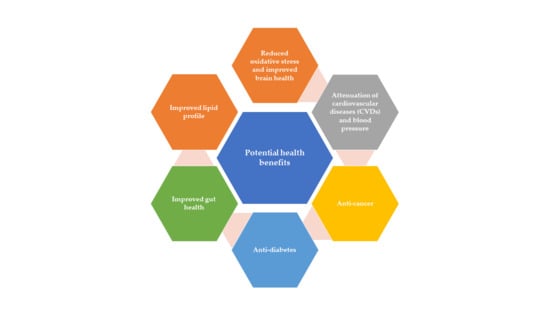
4. Promising Health Beneficial Attributes of Bioactive Components
4.1. Attenuation of Cardiovascular Diseases (CVDs) and Blood Pressure
4.2. Anti-Cancer
4.3. Anti-Diabetes
4.4. Gut Health
4.5. Lipids Profile Regulation
4.6. Oxidative Stress and Brain Health
5. Limitations/Risks
6. Concluding Remarks
7. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Healthy Diet (No. WHO-EM/NUT/282/E). World Health Organization. Regional Office for the Eastern Mediterranean. 2019. Available online: https://apps.who.int/iris/bitstream/handle/10665/325828/EMROPUB_2019_en_23536.pdf (accessed on 16 March 2021).
- Gürbüz, N.; Uluişik, S.; Frary, A.; Frary, A.; Doğanlar, S. Health benefits and bioactive compounds of eggplant. Food Chem. 2018, 268, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Dietary Bioactive Compounds and Their Health Implications. J. Food Sci. 2013, 78, A18–A25. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Šaponjac, V.T.; Čanadanović-Brunet, J.; Ćetković, G.; Djilas, S. Detection of Bioactive Compounds in Plants and Food Products. In Emerging and Traditional Technologies for Safe, Healthy and Quality Food; Springer: Cham, Switzerland, 2016; pp. 81–109. [Google Scholar] [CrossRef]
- Prakash, B.; Kujur, A.; Singh, P.P.; Kumar, A.; Yadav, A. Plants-derived bioactive compounds as functional food ingredients and food preservative. J. Nutr. Food Sci. 2017, 1, 1–7. Available online: https://www.henrypublishinggroups.com/wp-content/uploads/2018/01/plants-derived-bioactive-compounds-as-functional-food-ingredients-and-food-preservative.pdf (accessed on 5 November 2020).
- Plazas, M.; López-Gresa, M.P.; Vilanova, S.; Torres, C.; Hurtado, M.; Gramazio, P.; Andújar, I.; Herráiz, F.J.; Bellés, J.M.; Prohens, J. Diversity and Relationships in Key Traits for Functional and Apparent Quality in a Collection of Eggplant: Fruit Phenolics Content, Antioxidant Activity, Polyphenol Oxidase Activity, and Browning. J. Agric. Food Chem. 2013, 61, 8871–8879. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.P.; Kaur, A.; Shevkani, K.; Singh, N. Influence of jambolan (Syzygium cumini) and xanthan gum incorporation on the physicochemical, antioxidant and sensory properties of gluten-free eggless rice muffins. Int. J. Food Sci. Technol. 2015, 50, 1190–1197. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.-J.; Xu, D.-P.; Zhou, T.; Zhou, Y.; Li, S.; Li, H.-B. Bioactivities and Health Benefits of Wild Fruits. Int. J. Mol. Sci. 2016, 17, 1258. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.-J.; Li, Y.; Zhou, T.; Xu, D.-P.; Zhang, P.; Li, S.; Li, H.-B. Bioactivities and Health Benefits of Mushrooms Mainly from China. Molecules 2016, 21, 938. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Miguel, M.; Alonso, M.J.; Salaices, M.; Aleixandre, A.; López-Fandiño, R. Antihypertensive, ACE-inhibitory and vasodilator properties of an egg white hydrolysate: Effect of a simulated intestinal digestion. Food Chem. 2007, 104, 163–168. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-Derived Bioactive Peptides in Human Health: Challenges and Opportunities. Nutrient 2018, 10, 1738. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, R.; Meisel, H. Food-derived peptides with biological activity: From research to food applications. Curr. Opin. Biotechnol. 2007, 18, 163–169. [Google Scholar] [CrossRef]
- Aluko, R.E. Antihypertensive properties of plant-derived inhibitors of angiotensin I-converting enzyme activity: A review. Recent Prog. Med. Plants. 2008, 22, 541–561. [Google Scholar]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive peptides from marine processing waste and shellfish: A review. J. Funct. Foods 2012, 4, 6–24. [Google Scholar] [CrossRef]
- Cavazos, A.; De Mejia, E.G. Identification of Bioactive Peptides from Cereal Storage Proteins and Their Potential Role in Prevention of Chronic Diseases. Compr. Rev. Food Sci. Food Saf. 2013, 12, 364–380. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Fanning, K.; Netzel, M.; Turner, W.; Li, Y.; Schenk, P.M. Profiling of carotenoids and antioxidant capacity of microalgae from subtropical coastal and brackish waters. Food Chem. 2014, 165, 300–306. [Google Scholar] [CrossRef] [Green Version]
- Benke, K.K.; Benke, K.E. Experimental Design Issue for Assessment of Carotenoids Lutein and Zeaxanthin in Age-Related Eye Disease Study 2 Formulation for Age-Related Macular Degeneration. JAMA Ophthalmol. 2014, 132, 904. [Google Scholar] [CrossRef]
- Weikel, K.A.; Garber, C.; Baburins, A.; Taylor, A. Nutritional modulation of cataract. Nutr. Rev. 2014, 72, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, N.; Di Tomo, P.; Pandolfi, A. Carotenoids in cardiovascular disease prevention. JSM Atheroscler. 2016, 1, 1002. Available online: https://www.jscimedcentral.com/JSMAtherosclerosis/JSMAtherosclerosis-1-1002.pdf (accessed on 2 November 2020).
- Cooperstone, J.; Schwartz, S. Recent Insights into Health Benefits of Carotenoids. In Handbook on Natural Pigments in Food and Beverages; Woodhead Publishing: Cambridge, UK, 2016; pp. 473–497. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Casati, L.; Pagani, F.; Braga, P.C.; Scalzo, R.L.; Sibilia, V. Nasunin, a new player in the field of osteoblast protection against oxidative stress. J. Funct. Foods 2016, 23, 474–484. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, R.N.; Shiga, T.M.; Hassimotto, N.M.A.; Rosa, E.A.S.; Lajolo, F.M.; Cordenunsi, B.R. Phenolics and Antioxidant Properties of Fruit Pulp and Cell Wall Fractions of Postharvest Banana (Musa acuminata Juss.) Cultivars. J. Agric. Food Chem. 2010, 58, 7991–8003. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Bioactive compounds in banana and their associated health benefits—A review. Food Chem. 2016, 206, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gropper, S.S.; Smith, J.L. Advanced Nutrition and Human Metabolism Cengage Learning. 2012. Available online: https://www.cengage.com/c/advanced-nutrition-and-human-metabolism-6e-gropper/9781133104056PF/ (accessed on 22 October 2020).
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Erlund, I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr. Res. 2004, 24, 851–874. [Google Scholar] [CrossRef]
- Liggins, J.; Bluck, L.J.C.; Runswick, S.; Atkinson, C.; Coward, W.A.; Bingham, S.A. Daidzein and genistein contents of vegetables. Br. J. Nutr. 2000, 84, 717–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, S.; Joghataei, M.-T.; Mohseni, S.; Baluchnejadmojarad, T.; Bagheri, M.; Khamse, S.; Roghani, M. Naringenin improves learning and memory in an Alzheimer’s disease rat model: Insights into the underlying mechanisms. Eur. J. Pharmacol. 2015, 764, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Pereira-Caro, G.; Gaillet, S.; Ordóñez, J.L.; Mena, P.; Bresciani, L.; Bindon, K.A.; Del Rio, D.; Rouanet, J.-M.; Moreno-Rojas, J.M.; Crozier, A. Bioavailability of red wine and grape seed proanthocyanidins in rats. Food Funct. 2020, 11, 3986–4001. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Ortega, A.; Ordóñez, J.L.; Moreno-Rojas, R.; Moreno-Rojas, J.M.; Pereira-Caro, G. Changes in the Organosulfur and Polyphenol Compound Profiles of Black and Fresh Onion during Simulated Gastrointestinal Digestion. Foods 2021, 10, 337. [Google Scholar] [CrossRef]
- Moreno-Ortega, A.; Pereira-Caro, G.; Ordóñez, J.L.; Muñoz-Redondo, J.M.; Moreno-Rojas, R.; Pérez-Aparicio, J.; Moreno-Rojas, J.M. Changes in the antioxidant activity and metabolite profile of three onion varieties during the elaboration of ‘black onion’. Food Chem. 2020, 311, 125958. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Caro, G.; Clifford, M.N.; Polyviou, T.; Ludwig, I.A.; Alfheeaid, H.; Moreno-Rojas, J.M.; Garcia, A.L.; Malkova, D.; Crozier, A. Plasma pharmacokinetics of (poly)phenol metabolites and catabolites after ingestion of orange juice by endurance trained men. Free. Radic. Biol. Med. 2020, 160, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez-Díaz, J.L.; Hervalejo, A.; Pereira-Caro, G.; Muñoz-Redondo, J.M.; Romero-Rodríguez, E.; Arenas-Arenas, F.J.; Moreno-Rojas, J.M. Effect of Rootstock and Harvesting Period on the Bioactive Compounds and Antioxidant Activity of Two Orange Cultivars (‘Salustiana’ and ‘Sanguinelli’) Widely Used in Juice Industry. Processes 2020, 8, 1212. [Google Scholar] [CrossRef]
- Moreno-Ortega, A.; Pereira-Caro, G.; Ordóñez, J.L.; Moreno-Rojas, R.; Ortíz-Somovilla, V.; Moreno-Rojas, J.M. Bioaccessibility of Bioactive Compounds of ‘Fresh Garlic’ and ‘Black Garlic’ through In Vitro Gastrointestinal Digestion. Foods 2020, 9, 1582. [Google Scholar] [CrossRef]
- Quintero-Flórez, A.; Pereira-Caro, G.; Sánchez-Quezada, C.; Moreno-Rojas, J.M.; Gaforio, J.J.; Jimenez, A.; Beltrán, G. Effect of olive cultivar on bioaccessibility and antioxidant activity of phenolic fraction of virgin olive oil. Eur. J. Nutr. 2018, 57, 1925–1946. [Google Scholar] [CrossRef]
- Majid, A.; Priyadarshini, C.G.P. Millet derived bioactive peptides: A review on their functional properties and health benefits. Crit. Rev. Food Sci. Nutr. 2020, 60, 3342–3351. [Google Scholar] [CrossRef] [PubMed]
- Dias-Martins, A.M.; Pessanha, K.L.F.; Pacheco, S.; Rodrigues, J.A.S.; Carvalho, C.W.P. Potential use of pearl millet (Pennisetum glaucum (L.) R. Br.) in Brazil: Food security, processing, health benefits and nutritional products. Food Res. Int. 2018, 109, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Udenigwe, C.C.; Aluko, R.E. Food Protein-Derived Bioactive Peptides: Production, Processing, and Potential Health Benefits. J. Food Sci. 2012, 77, R11–R24. [Google Scholar] [CrossRef]
- Moreno-Rojas, J.M.; Moreno-Ortega, A.; Ordóñez, J.L.; Moreno-Rojas, R.; Pérez-Aparicio, J.; Pereira-Caro, G. Development and validation of UHPLC-HRMS methodology for the determination of flavonoids, amino acids and organosulfur compounds in black onion, a novel derived product from fresh shallot onions (Allium cepa var. aggregatum). LWT 2018, 97, 376–383. [Google Scholar] [CrossRef]
- World Health Organization. Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases/#tab=tab_1 (accessed on 3 November 2020).
- Călinoiu, L.F.; Vodnar, D.C. Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef] [Green Version]
- Sanjukta, S.; Rai, A.K. Production of bioactive peptides during soybean fermentation and their potential health benefits. Trends Food Sci. Technol. 2016, 50, 1–10. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rodrigues, C.F.; Sharopov, F.; Docea, A.O.; Karaca, A.C.; Sharifi-Rad, M.; Karıncaoglu, D.K.; Gülseren, G.; Özçelik, B.; Demircan, E.; et al. Diet, Lifestyle and Cardiovascular Diseases: Linking Pathophysiology to Cardioprotective Effects of Natural Bioactive Compounds. Int. J. Environ. Res. Public Health 2020, 17, 2326. [Google Scholar] [CrossRef] [Green Version]
- Cicero, A.F.G.; Fogacci, F.; Colletti, A. Food and plant bioactives for reducing cardiometabolic disease risk: An evidence based approach. Food Funct. 2017, 8, 2076–2088. [Google Scholar] [CrossRef]
- Patten, G.S.; Abeywardena, M.Y.; Bennett, L.E. Inhibition of Angiotensin Converting Enzyme, Angiotensin II Receptor Blocking, and Blood Pressure Lowering Bioactivity across Plant Families. Crit. Rev. Food Sci. Nutr. 2016, 56, 181–214. [Google Scholar] [CrossRef] [PubMed]
- Varzakas, T.; Zakynthinos, G.; Verpoort, F. Plant Food Residues as a Source of Nutraceuticals and Functional Foods. Foods 2016, 5, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barera, A.; Buscemi, S.; Monastero, R.; Caruso, C.; Caldarella, R.; Ciaccio, M.; Vasto, S. β-glucans: Ex vivo inflammatory and oxidative stress results after pasta intake. Immun. Ageing 2016, 13, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyama, M.; Naramoto, K.; Nakajima, T.; Aoyama, T.; Watanabe, M.; Nakamura, K. Purification and Identification of Antihypertensive Peptides from Fermented Buckwheat Sprouts. J. Agric. Food Chem. 2013, 61, 3013–3021. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.R.S.; González, F.P.; Guerrero, L.C.; Ancona, D.B. Angiotensin I-Converting Enzyme Inhibitory Peptides of Chia (Salvia hispanica) Produced by Enzymatic Hydrolysis. Int. J. Food Sci. 2013, 2013, 158482. [Google Scholar] [CrossRef] [Green Version]
- Toufektsian, M.-C.; De Lorgeril, M.; Nagy, N.; Salen, P.; Donati, M.B.; Giordano, L.; Mock, H.-P.; Peterek, S.; Matros, A.; Petroni, K.; et al. Chronic Dietary Intake of Plant-Derived Anthocyanins Protects the Rat Heart against Ischemia-Reperfusion Injury. J. Nutr. 2008, 138, 747–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tejada, S.; Pinya, S.; Bibiloni, M.D.M.; Tur, J.A.; Pons, A.; Sureda, A. Cardioprotective Effects of the Polyphenol Hydroxytyrosol from Olive Oil. Curr. Drug Targets 2017, 18, 1477–1486. [Google Scholar] [CrossRef]
- Afshari, F.; Seraj, H.; Hashemi, Z.S.; Timajchi, M.; Ensiyeh, O.; Ladan, G.; Asadi, M.; Elyasi, Z.; GanjiBakhsh, M. The Cytotoxic Effects of Eggplant Peel Extract on Human Gastric Adenocarcinoma Cells and Normal Cells. Mod. Med Lab. J. 2017, 1, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Shen, K.-H.; Hung, J.-H.; Chang, C.-W.; Weng, Y.-T.; Wu, M.-J.; Chen, P.-S. Solasodine inhibits invasion of human lung cancer cell through downregulation of miR-21 and MMPs expression. Chem. Interact. 2017, 268, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Montales, M.T.E.; Rahal, O.M.; Kang, J.; Rogers, T.J.; Prior, R.L.; Wu, X.; Simmen, R.C. Repression of mammosphere formation of human breast cancer cells by soy isoflavone genistein and blueberry polyphenolic acids suggests diet-mediated targeting of cancer stem-like/progenitor cells. Carcinogenesis 2012, 33, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yao, C.; Chen, X.; Xia, H.; Zhang, L.; Liu, H.; Jiang, X.; Dai, Y.; Liu, J. The Fruits of Maclura pomifera Extracts Inhibits Glioma Stem-Like Cell Growth and Invasion. Neurochem. Res. 2013, 38, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.-K.; Wu, A.T.H.; Lee, W.-H.; Chang, T.-C.; Chiou, J.-F.; Wang, L.-S.; Wu, C.-H.; Huang, C.-Y.F.; Shieh, Y.-S.; Chao, T.-Y.; et al. Pterostilbene, a bioactive component of blueberries, suppresses the generation of breast cancer stem cells within tumor microenvironment and metastasis via modulating NF-κB/microRNA 448 circuit. Mol. Nutr. Food Res. 2013, 57, 1123–1134. [Google Scholar] [CrossRef]
- Leung, E.H.W.; Ng, T.B. A relatively stable antifungal peptide from buckwheat seeds with antiproliferative activity toward cancer cells. J. Pept. Sci. 2007, 13, 762–767. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Miralles, B.; Carrillo, W.; Hernández-Ledesma, B. In vitro chemopreventive properties of peptides released from quinoa (Chenopodium quinoa Willd.) protein under simulated gastrointestinal digestion. Food Res. Int. 2018, 105, 403–411. [Google Scholar] [CrossRef]
- Faria, A.; Pestana, D.; Teixeira, D.; De Freitas, V.; Mateus, N.; Calhau, C. Blueberry anthocyanins and pyruvic acid adducts: Anticancer properties in breast cancer cell lines. Phytother. Res. 2010, 24, 1862–1869. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-H.; Lee, J.; Herald, T.; Cox, S.; Noronha, L.; Perumal, R.; Lee, H.-S.; Smolensky, D. Anticancer Activity of a Novel High Phenolic Sorghum Bran in Human Colon Cancer Cells. Oxidative Med. Cell. Longev. 2020, 2020, 2890536. [Google Scholar] [CrossRef]
- Cheng, D.M.; Pogrebnyak, N.; Kuhn, P.; Poulev, A.; Waterman, C.; Rojas-Silva, P.; Johnson, W.D.; Raskin, I. Polyphenol-rich Rutgers Scarlet Lettuce improves glucose metabolism and liver lipid accumulation in diet-induced obese C57BL/6 mice. Nutrients 2014, 30, S52–S58. [Google Scholar] [CrossRef] [Green Version]
- Vilcacundo, R.; Martínez-Villaluenga, C.; Hernández-Ledesma, B. Release of dipeptidyl peptidase IV, α-amylase and α-glucosidase inhibitory peptides from quinoa (Chenopodium quinoa Willd.) during in vitro simulated gastrointestinal digestion. J. Funct. Foods 2017, 35, 531–539. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.J.; Kim, H.J.; Kim, M.J.; Kang, S.; Kim, D.S.; Daily, J.W.; Jeong, D.Y.; Kwon, D.Y.; Park, S. Standardized chungkookjang, short-term fermented soybeans with Bacillus lichemiformis, improves glucose homeostasis as much as traditionally made chungkookjang in diabetic rats. J. Clin. Biochem. Nutr. 2012, 12–54. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified Anthocyanin Supplementation Reduces Dyslipidemia, Enhances Antioxidant Capacity, and Prevents Insulin Resistance in Diabetic Patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Liu, Y.; Sun, B. Peptides Derived from Oats Improve Insulin Sensitivity and Lower Blood Glucose in Streptozotocin-Induced Diabetic Mice. J. Biomed. Sci. 2015, 4. [Google Scholar] [CrossRef]
- Wang, J.; Du, K.; Fang, L.; Liu, C.; Min, W.; Liu, J. Evaluation of the antidiabetic activity of hydrolyzed peptides derived from Juglans mandshurica Maxim. fruits in insulin-resistant HepG2 cells and type 2 diabetic mice. J. Food Biochem. 2018, 42, e12518. [Google Scholar] [CrossRef]
- Barik, S.K.; Russell, W.R.; Moar, K.M.; Cruickshank, M.; Scobbie, L.; Duncan, G.; Hoggard, N. The anthocyanins in black currants regulate postprandial hyperglycaemia primarily by inhibiting α-glucosidase while other phenolics modulate salivary α-amylase, glucose uptake and sugar transporters. J. Nutr. Biochem. 2020, 78, 108325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, X.; Sun, Y.; Hu, B.; Sun, Y.; Jabbar, S.; Zeng, X. Fermentation in vitro of EGCG, GCG and EGCG3”Me isolated from Oolong tea by human intestinal microbiota. Food Res. Int. 2013, 54, 1589–1595. [Google Scholar] [CrossRef]
- Chacar, S.; Itani, T.; Hajal, J.; Saliba, Y.; Louka, N.; Faivre, J.-F.; Maroun, R.; Fares, N. The Impact of Long-Term Intake of Phenolic Compounds-Rich Grape Pomace on Rat Gut Microbiota. J. Food Sci. 2018, 83, 246–251. [Google Scholar] [CrossRef]
- Han, M.; Song, P.; Huang, C.; Rezaei, A.; Farrar, S.; Brown, M.A.; Ma, X. Dietary grape seed proanthocyanidins (GSPs) improve weaned intestinal microbiota and mucosal barrier using a piglet model. Oncotarget 2016, 7, 80313–80326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef] [Green Version]
- Nagata, R.; Echizen, M.; Yamaguchi, Y.; Han, K.-H.; Shimada, K.; Ohba, K.; Kitano-Okada, T.; Nagura, T.; Uchino, H.; Fukushima, M. Effect of a combination of inulin and polyphenol-containing adzuki bean extract on intestinal fermentation in vitro and in vivo. Biosci. Biotechnol. Biochem. 2018, 82, 489–496. [Google Scholar] [CrossRef]
- Kim, J.; Choi, J.H.; Ko, G.; Jo, H.; Oh, T.; Ahn, B.; Unno, T. Anti-Inflammatory Properties and Gut Microbiota Modulation of Porphyra tenera Extracts in Dextran Sodium Sulfate-Induced Colitis in Mice. Antioxidants 2020, 9, 988. [Google Scholar] [CrossRef]
- Basuny, A.M.; Arafat, S.M.; El-Marzooq, M.A. Antioxidant and antihyperlipidemic activities of anthocyanins from egg-plant peels. J. Pharma Res. Rev. 2012, 2, 50–57. [Google Scholar]
- Vijayakumar, S.; Presannakumar, G.; Vijayalakshmi, N.R. Investigations on the Effect of Flavonoids from Banana, Musa Paradisiaca L. on Lipid Metabolism in Rats. J. Diet. Suppl. 2009, 6, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Halaby, M.S.; Metwally, E.M.; Omar, A.A. Effect of Moringa oleifera on serum lipids and kidney function of hyperlipidemic rats. J. Appl. Sci. Res. 2013, 9, 5189–5198. [Google Scholar]
- Neyrinck, A.M.; Van Hée, V.F.; Bindels, L.B.; De Backer, F.; Cani, P.; Delzenne, N.M. Polyphenol-rich extract of pomegranate peel alleviates tissue inflammation and hypercholesterolaemia in high-fat diet-induced obese mice: Potential implication of the gut microbiota. Br. J. Nutr. 2013, 109, 802–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Sun, Y.-R.; He, L.-B.; Huang, L.; Li, T.-T.; Wang, T.-Y.; Tang, L. Amelioration by Idesia polycarpa Maxim. var. vestita Diels. of Oleic Acid-Induced Nonalcoholic Fatty Liver in HepG2 Cells through Antioxidant and Modulation of Lipid Metabolism. Oxidative Med. Cell. Longev. 2020, 2020, 1208726. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, S.; Rajan, T.S.; De Nicola, G.R.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. The Isothiocyanate Isolated from Moringa oleifera Shows Potent Anti-Inflammatory Activity in the Treatment of Murine Subacute Parkinson’s Disease. Rejuvenation Res. 2017, 20, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.P.; Luz, D.A.; da Silva, C.C.S.; Malcher, C.M.R.; Fernandes, L.M.P.; Santa, H.S.D.; Gomes, A.R.Q.; Monteiro, M.C.; Ribeiro, C.H.M.A.; Fontes-Júnior, E.A.; et al. Ganoderma lucidum Ameliorates Neurobehavioral Changes and Oxidative Stress Induced by Ethanol Binge Drinking. Oxidative Med. Cell. Longev. 2020, 2020, 2497845. [Google Scholar] [CrossRef] [PubMed]
- Wattanathorn, J.; Palachai, N.; Thukham-Mee, W.; Muchimapura, S. Memory-Enhancing Effect of a Phytosome Containing the Combined Extract of Mulberry Fruit and Ginger in an Animal Model of Ischemic Stroke with Metabolic Syndrome. Oxidative Med. Cell. Longev. 2020, 2020, 3096826. [Google Scholar] [CrossRef]
- Yang, D.; Jiang, Y.; Wang, Y.; Lei, Q.; Zhao, X.; Yi, R.; Zhang, X. Improvement of Flavonoids in Lemon Seeds on Oxidative Damage of Human Embryonic Kidney 293T Cells Induced by H2O2. Oxidative Med. Cell. Longev. 2020, 2020, 3483519. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, I.; Kobayashi, M.; Hamada, T.; Tsuda, K.; Goto, H.; Imaizumi, K.; Nozawa, A.; Sugimoto, A.; Kakuda, T. Heat-Epimerized Tea Catechins Rich in Gallocatechin Gallate and Catechin Gallate are More Effective to Inhibit Cholesterol Absorption than Tea Catechins Rich in Epigallocatechin Gallate and Epicatechin Gallate. J. Agric. Food Chem. 2003, 51, 7303–7307. [Google Scholar] [CrossRef] [PubMed]
- Barona, J.; Aristizabal, J.C.; Blesso, C.N.; Volek, J.S.; Fernandez, M.L. Grape Polyphenols Reduce Blood Pressure and Increase Flow-Mediated Vasodilation in Men with Metabolic Syndrome. J. Nutr. 2012, 142, 1626–1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pistollato, F.; Giampieri, F.; Battino, M. The use of plant-derived bioactive compounds to target cancer stem cells and modulate tumor microenvironment. Food Chem. Toxicol. 2015, 75, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wicha, M.S.; Schwartz, S.J.; Sun, D. Implications of cancer stem cell theory for cancer chemoprevention by natural dietary compounds. J. Nutr. Biochem. 2011, 22, 799–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Berghe, W.V. Epigenetic impact of dietary polyphenols in cancer chemoprevention: Lifelong remodeling of our epigenomes. Pharmacol. Res. 2012, 65, 565–576. [Google Scholar] [CrossRef]
- Prasad, S.; Phromnoi, K.; Yadav, V.R.; Chaturvedi, M.M.; Aggarwal, B.B. Targeting Inflammatory Pathways by Flavonoids for Prevention and Treatment of Cancer. Planta Med. 2010, 76, 1044–1063. [Google Scholar] [CrossRef] [Green Version]
- Salminen, A.; Lehtonen, M.; Suuronen, T.; Kaarniranta, K.; Huuskonen, J. Terpenoids: Natural inhibitors of NF-κB signaling with anti-inflammatory and anticancer potential. Cell. Mol. Life Sci. 2008, 65, 2979–2999. [Google Scholar] [CrossRef]
- Dobani, S.; Latimer, C.; McDougall, G.J.; Allwood, J.W.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Ternan, N.G.; Pourshahidi, L.K.; Lawther, R.; Tuohy, K.M.; et al. Ex vivo fecal fermentation of human ileal fluid collected after raspberry consumption modifies (poly)phenolics and modulates genoprotective effects in colonic epithelial cells. Redox Biol. 2021, 40, 101862. [Google Scholar] [CrossRef]
- Henidi, H.A.; Al-Abbasi, F.A.; El-Moselhy, M.A.; El-Bassossy, H.M.; Al-Abd, A.M. Despite Blocking Doxorubicin-Induced Vascular Damage, Quercetin Ameliorates Its Anti breast Cancer Activity. Oxidative Med. Cell. Longev. 2020, 2020, 8157640. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.P.; Goswami, A.; Kalia, K.; Kate, A.S. Plant-Derived Bioactive Peptides: A Treatment to Cure Diabetes. Int. J. Pept. Res. Ther. 2020, 26, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Ji, M.; Xu, J.; Zhang, C.; Li, M. Hypoglycemic effects of bioactive ingredients from medicine food homology and medicinal health food species used in China. Crit. Rev. Food Sci. Nutr. 2020, 60, 2303–2326. [Google Scholar] [CrossRef]
- Yang, L.; Shu, L.; Yao, D.D.; Jia, X.B.; Yu, S.M. Study on the glucose-lowering effect of puerarin in STZ-induced diabetic mice. Chin. J. Hosp. Pharm. 2014, 34, 1338–1342. [Google Scholar]
- Wan, M.L.Y.; Ling, K.H.; El-Nezami, H.; Wang, M.F. Influence of functional food components on gut health. Crit. Rev. Food Sci. Nutr. 2019, 59, 1927–1936. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability—A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo-Liberona, N.; González-Domínguez, R.; Vegas, E.; Riso, P.; Del Bo’, C.; Bernardi, S.; Peron, G.; Guglielmetti, S.; Gargari, G.; Kroon, P.A.; et al. Increased Intestinal Permeability in Older Subjects Impacts the Beneficial Effects of Dietary Polyphenols by Modulating Their Bioavailability. J. Agric. Food Chem. 2020, 68, 12476–12484. [Google Scholar] [CrossRef]
- Loo, Y.T.; Howell, K.; Chan, M.; Zhang, P.; Ng, K. Modulation of the human gut microbiota by phenolics and phenolic fiber-rich foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1268–1298. [Google Scholar] [CrossRef]
- Dey, P. Gut microbiota in phytopharmacology: A comprehensive overview of concepts, reciprocal interactions, biotransformations and mode of actions. Pharmacol. Res. 2019, 147, 104367. [Google Scholar] [CrossRef]
- Gong, L.; Cao, W.; Chi, H.; Wang, J.; Zhang, H.; Liu, J.; Sun, B. Whole cereal grains and potential health effects: Involvement of the gut microbiota. Food Res. Int. 2018, 103, 84–102. [Google Scholar] [CrossRef]
- Siasos, G.; Tousoulis, D.; Tsigkou, V.; Kokkou, E.; Oikonomou, E.; Vavuranakis, M.; Basdra, E.; Papavassiliou, A.; Stefanadis, C. Flavonoids in Atherosclerosis: An Overview of Their Mechanisms of Action. Curr. Med. Chem. 2013, 20, 2641–2660. [Google Scholar] [CrossRef]
- Vergara-Jimenez, M.; AlMatrafi, M.M.; Fernandez, M.L. Bioactive Components in Moringa Oleifera Leaves Protect against Chronic Disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Peanut (Arachis hypogaea L.): A Prospective Legume Crop to Offer Multiple Health Benefits under Changing Climate. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1325–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marangoni, F.; Poli, A. Phytosterols and cardiovascular health. Pharmacol. Res. 2010, 61, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Galvez, A.F.U.S. Products and Methods Using Soy Peptides to Lower Total and LDL Cholesterol Levels; U.S. Patent No. 8,598,111; U.S. Patent and Trademark Office: Washington, DC, USA, 2013; Available online: https://patentimages.storage.googleapis.com/2d/c5/02/1ab4f01370c428/US8598111.pdf (accessed on 22 November 2020).
- Xu, J.; Ma, C.; Han, L.; Gao, H.; Zhou, Q.; Yang, M.; Chen, C.; Deng, Q.; Huang, Q.; Huang, F. Optimized rapeseed oils rich in endogenous micronutrients ameliorate risk factors of atherosclerosis in high fat diet fed rats. Lipids Health Dis. 2014, 13, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welcome, M.O. Neuroinflammation in CNS diseases: Molecular mechanisms and the therapeutic potential of plant derived bioactive molecules. PharmaNutrition 2020, 11, 100176. [Google Scholar] [CrossRef]
- Piemontese, L. Plant Food Supplements with Antioxidant Properties for the Treatment of Chronic and Neurodegenerative Diseases: Benefits or Risks? J. Diet. Suppl. 2017, 14, 478–484. [Google Scholar] [CrossRef]
- Lu, M.; Tan, L.; Zhou, X.-G.; Yang, Z.-L.; Zhu, Q.; Chen, J.-N.; Luo, H.-R.; Wu, G.-S. Secoisolariciresinol Diglucoside Delays the Progression of Aging-Related Diseases and Extends the Lifespan of Caenorhabditis elegans via DAF-16 and HSF-1. Oxidative Med. Cell. Longev. 2020, 2020, 1293935. [Google Scholar] [CrossRef]
- Libro, R.; Giacoppo, S.; Rajan, T.S.; Bramanti, P.; Mazzon, E. Natural Phytochemicals in the Treatment and Prevention of Dementia: An Overview. Molecules 2016, 21, 518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regitz, C.; Fitzenberger, E.; Mahn, F.L.; Dußling, L.M.; Wenzel, U. Resveratrol reduces amyloid-beta (Aβ1–42)-induced paralysis through targeting proteostasis in an Alzheimer model of Caenorhabditis elegans. Eur. J. Nutr. 2016, 55, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Presannakumar, G.; Vijayalakshmi, N. Antioxidant activity of banana flavonoids. Fitoterapia 2008, 79, 279–282. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Polyviou, T.; Ludwig, I.A.; Nastase, A.-M.; Moreno-Rojas, J.M.; Garcia, A.L.; Malkova, D.; Crozier, A. Bioavailability of orange juice (poly)phenols: The impact of short-term cessation of training by male endurance athletes. Am. J. Clin. Nutr. 2017, 106, 791–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quesada-Gómez, J.M.; Santiago-Mora, R.; Durán-Prado, M.; Dorado, G.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Casado-Díaz, A. β-Cryptoxanthin Inhibits Angiogenesis in Human Umbilical Vein Endothelial Cells Through Retinoic Acid Receptor. Mol. Nutr. Food Res. 2017, 62, 1700489. [Google Scholar] [CrossRef] [PubMed]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [Green Version]
- Gülçin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Leuci, R.; Brunetti, L.; Poliseno, V.; Laghezza, A.; Loiodice, F.; Tortorella, P.; Piemontese, L. Natural Compounds for the Pre-vention and Treatment of Cardiovascular and Neurodegenerative Diseases. Foods 2021, 10, 29. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anti-cancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef]
- Noce, A.; Di Lauro, M.; Di Daniele, F.; Zaitseva, A.P.; Marrone, G.; Borboni, P.; Di Daniele, N. Natural Bioactive Compounds Useful in Clinical Management of Metabolic Syndrome. Nutrients 2021, 13, 630. [Google Scholar] [CrossRef] [PubMed]




| Plant Food | Bioactive Components | Treatment | Health Benefits | References |
|---|---|---|---|---|
| Buckwheat | Bioactive peptides | Single oral dose of 0.010 mg/kg BW administered in spontaneously hypertensive rats; blood pressure was recorded at 0, 3, 6, 9, and 24 h after administration. | Lowering of blood pressure | [53] |
| Chia seeds | Bioactive peptides | Measurement of ACE-I inhibitory activity through regression analysis of ACE-I inhibitory activity (%) vs. peptide concentration, and IC50 values (in vitro enzymatic activity) | ACE-inhibitory activity (Blood pressure) | [54] |
| Maize seed | Anthocyanins | 8 weeks anthocyanin-free (ACN-free) or anthocyanin-rich (ACN-rich) diets were fed to male Wistar rats | Reduction of cardiovascular disease | [55] |
| Olive oil | Polyphenols | Randomized 50 participants ingested 60 mL/day (polyphenol olive oil (360 mg/kg polyphenols)) | Reduction of cardiovascular disease | [56] |
| Eggplant | Eggplant extract | Using different concentrations of 10, 5, 2.5, 1.25, 0.6, 0.3, 0.15, 0.06, 0.03, 0.01 mg/mL MTT test was performed using cells for 48 h. | Toxic effect on cancer cells | [57] |
| Eggplant | Glycoalkaloids (solasodine) | Cells (A549) were seeded in a 96-well plate and treated with solasodine in triplicate | Anti-cancer activity | [58] |
| Soy | Isoflavone genistein | Genistein concentration range (40 nM and 2 μM) were tested using MDA-MB-231 and MCF-7 | Anti-cancer activity | [59] |
| Maclura pomifera | Pomiferin (flavonoid extracts) | Using the MTS assay (1 and 10 μM); inhibits the both U373 and U87 neurosphere cells growth | Anti-cancer activity | [60] |
| Blueberries | Pterostilbene | Pterostilbene concentration range (2.5, 5, and 10 μM) was added 6 h after seeding MDA-MB-231 or MCF7 cells; MDA-MB-231 cells were cocultured with M2 TAM and subcutaneously injected into the NOD/SCID mice), pterostilbene-treated and control groups (5 mg/kg, ip injection, 5 times/week). | Anti-cancer activity | [61] |
| Buckwheat | Bioactive peptide (4 kDa peptide) | Cancerous cell lines L1210, HepG2, MCF 7, and WRL68 were seeded into each well of a 96-well culture plate (treated with various concentration) | Anti-cancer activity | [62] |
| Quinoa | Bioactive peptides | Caco-2, HCT-116 and HT-29 cells were seeded in 96-well Plates; quinoa protein concentrate digests, fractions or blanks at different concentrations (4 to 0.031 mg/mL) for 24 h | Anti-cancer activity | [63] |
| Blueberry | Anthocyanins | MDAMB-231 and MCF-7 cells were used; anthocyanin-pyruvic acid adduct extract and blueberry anthocyanin extract at 250 μg/mL, for 24 h | Anti-cancer activity | [64] |
| Sorghum | Phenolic (sorghum bran extract) | Human colon cancer cells were cultured in Dulbecco’s modified Eagle medium; cells treated with extract of phenolic sorghum bran having concentration 0, 0.625, 1.25, 2.5, 5.0, and 10.0 mg/mL. (diluted 70% ethanol + 5% citric acid) | Anti-cancer activity | [65] |
| Lettuce | High polyphenol content | For 28 days vehicle (water), Metformin (250 mg/kg) or Rutgers Scarlet Lettuce extract (100 or 300 mg/kg) were orally administered to obese mice (high fat diet-induced) | Anti-diabetic effects | [66] |
| Quinoa | Bioactive peptides | a-amylase inhibition assay, negative control (distilled water) or positive control (2 mM acarbose), 50 µL of sample, added to 100 µL a-amylase solution; a-glucosidase inhibition assay, 100 mL of sample, negative control (distilled water) or positive control (1 mM acarbose) were added to 50 mL of rat intestine a-glucosidase | Anti-diabetic effects | [67] |
| Soybean | Isoflavonoid aglycones and small peptides | Type 2 diabetic rats were administered 10% cooked soyabeamns, 10% traditional chungkookjang, or standardized chungkookjang fermented with Bacillus lichemiformis (for 8 weeks) | Improved insulinotropic activity | [68] |
| Bilberry and blackcurrant | Anthocyanin | For 24 weeks 58 diabetic patients were fed with 160 mg of anthocyanins two times/day or placebo (n = 29/group) | Anti-diabetic effects | [69] |
| Oat seed | Bioactive peptides (proteins hydrolysate) | For four weeks diabetic mice groups were orally supplemented with high oat peptide, medium oat peptide and low oat peptide 1.0, 0.5 and 0.25 g/kg body weight in the solutions containing 0.6, 0.3 and 0.15 g mL−1 oat peptides solution, respectively. | Anti-diabetic effects (inhibits alpha-glucosidase enzyme) | [70] |
| Juglans mandshurica(Manchurian walnut) | Walnut hydrolyzed peptides | Diabetic mice administered Met hydrochloride 125 mg/kg/day; WHPs (3–10 kDa molecular weight) 200 mg/kg/day; WHPs (3–10 kDa molecular weight) 500 mg/kg/day; and WHPs (3–10 kDa molecular weight) 800 mg/kg/day (for 4 weeks) | Anti-diabetic activity | [71] |
| Black currants | Anthocyanins | α-amylase, α-glucosidase assay (in vitro) using 96-well plate format | Anti-diabetic activity (type-2 diabetes) | [72] |
| Oolong tea | (−)-Epigallocatechin gallate, (+)-gallocatechin gallate, and (−)-epigallocatechin | EGCG, GCG and EGCG3”Me on human intestinal microbiota in vitro were evaluated by monitoring bacterial populations; Fermentation was initiated by adding 150 μL of fecal slurry to 1350 μL of culture medium containing EGCG, GCG, EGCG3”Me or FOS (positive control) | Gut health (promoted the growth of beneficial bacteria) | [73] |
| Grape | Grape pomace extract (phenolic compounds-PC) | For 14 months, diets of four groups (2 months old rats) were supplemented with various PC concentrations (diluted in 0.1% DMSO), 2.5, 5, 10, and 20 mg/kg/d) and 1 group administered 0.1% Dimethyl Sulfoxide (DMSO) alone (control group) | Gut health | [74] |
| Grape | Proanthocyanidins | For 28 days, four groups of weaned piglets (28 days) were fed a control diet, or administered with 250 mg/kg proanthocyanidins and half-dose antibiotics or 250 mg/kg proanthocyanidins, kitasamycin/colistin. | Gut health | [75] |
| Cranberry | Polyphenols | For 8 weeks, high fat high sugar mice were orally intubated with cranberry extract (CE) (200 mg/kg) or vehicle (water) | Gut health | [76] |
| Adzuki bean | Polyphenols | Through in vitro (48 h) and in vivo (rats) 28 day study, effects of inulin (INU) and polyphenol-containing adzuki bean extract (AE) on intestinal fermentation were evaluated; final concentrations of pig feces, AE, nitrogen source and carbohydrates in the fermenters were 2, 1, 0.8, and 3% (w/v), respectively; experimental diets based on the AIN-93G diet (INU + 4% AE; INU + 1% AE; INU; CEL + 4% AE; CEL + 1% AE; and CEL), CEL-High cellulose | Gut health | [77] |
| Porphyra tenera(PT) seaweed food | Porphyra tenera (PT) extract | Colitis-induced mice model was used; for 1 week DSS was administered using drinking water, and in mice gastrointestinal tract PT extract was ingested. | Gut health | [78] |
| Eggplant | Anthocyanins | 8 week study; six groups of rats were formed and one group was fed a standard pellet diet, other groups fed with high-fat diet; 3 groups (3–5) given 200, 400 and 800 ppm of eggplant peel anthocyanins and group 6 received tertiary butylhydroquinone (TBHQ) (200 ppm). | Reduction of cholesterol levels | [79] |
| M. paradisiaca | Flavonoids | Male rats were administered extracted flavonoids at a dose of 1 mg/100 g (BW)/day | Reduction of cholesterol levels (hypolipidemic activities) | [80] |
| Moringa Oleifera | Leaves powder | Rats were fed for 45 days; Group 1: basal diet; Group 2: (positive control) basal diet, in addition 2% cholesterol to induce (hyperlipidemia); Group 3 and group 4: same composition as positive diet, in addition fortified bread with 10% & 15% MO leaves powder | Cholesterol lowering effects | [81] |
| Pomegranate | Pomegranate peel extract | 4 weeks study period; male Balb/c mice were divided into three groups: a control (CT) group, one fed with HF diet and other group fed the same HF diet and also given pomegranate peel extract at a dose of 0·2% (6 mg/d per mouse). | Reduce serum cholesterol level | [82] |
| Idesia polycarpa(edible oil plant) | Polyphenols | TGs were measured using an enzymatic method kit using HepG2 cell | Lipid-lowering effect | [83] |
| Moringa Oleifera | Isothyocynate | For 1 week mice were pretreated with moringin (10 mg/kg + 5 μL myrosinase/mouse/day) and with GMG (10 mg/kg). | Improved oxidative stress and brain health | [84] |
| Ganoderma lucidum | Aqueous Extract of G. lucidum (AEGI) | Beginning at 35 days old, Wistar rats were exposed to 5 binges of ethanol (3 g/kg/day); after 24 h of last binge supplementation, for three consecutive days AEGl (100 mg/kg/day) or distilled water were given to animals | Improve brain health | [85] |
| Mulberry fruit and ginger | Combined extract (PMG) | A 16 week feeding study of high-carbohydrate high-fat (Wistar rats). PMG was orally administered 21 days before and 21 days after the occlusion of the right middle cerebral artery to MetS rats at doses of 50, 100, and 200 mg·kg−1 BW | Improvement in oxidative stress and brain health | [86] |
| Lemon seeds | Flavonoids | Different flavonoid concentrations (150 μg/mL 100 μg/mL and 50 μg/mL) were used to treat HEK 293T cells | Improvement in oxidative damage | [87] |
| Tea | Gallocatechin gallate | Cholesterol micellar solubility of was determined (Using catechins 1 and 2 mM), Lymphatic Recovery of 14C-Cholesterol in Rats Cannulated in the Thoracic Duct performed (using 100 mg and 120 mg in 3 mL catechins emulsion) | Reduction of cholesterol | [88] |
| Grape | Polyphenols | Double-blind, crossover study, 30 days consumption of freeze-dried grape polyphenol powder or a placebo | Reduction of cardiovascular disease | [89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. https://doi.org/10.3390/foods10040839
Samtiya M, Aluko RE, Dhewa T, Moreno-Rojas JM. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods. 2021; 10(4):839. https://doi.org/10.3390/foods10040839
Chicago/Turabian StyleSamtiya, Mrinal, Rotimi E. Aluko, Tejpal Dhewa, and José Manuel Moreno-Rojas. 2021. "Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview" Foods 10, no. 4: 839. https://doi.org/10.3390/foods10040839
APA StyleSamtiya, M., Aluko, R. E., Dhewa, T., & Moreno-Rojas, J. M. (2021). Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods, 10(4), 839. https://doi.org/10.3390/foods10040839