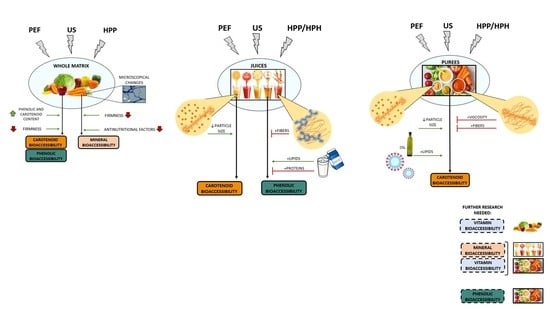
Recent Advances toward the Application of Non-Thermal Technologies in Food Processing: An Insight on the Bioaccessibility of Health-Related Constituents in Plant-Based Products
Abstract
:1. Introduction
2. Factors Affecting Bioaccessibility of Bioactive Compounds and Micronutrients
2.1. Carotenoids
2.2. Phenolic Compounds
2.3. Minerals
2.4. Vitamins
3. Impact of Non-Thermal Processing Technologies on Bioaccessibility
3.1. Carotenoids
3.2. Phenolic Compounds
3.3. Minerals
3.4. Vitamins
| Food Matrix | Processing Conditions | Structure | Micronutrients Content | Bioaccessibility Increase | Bioaccessibility Decrease | References |
|---|---|---|---|---|---|---|
| Apple | High pressure processing (HPP) (500 MPa for 2, 4, 8 and 10 min) | No information provided about structure | ↑ Calcium (2–8 min) ↓ Zinc (2, 4 min) ↑ Zinc (10 min) | Iron (8 min) Zinc (8, 10 min) | Calcium (all treatments) Iron (4 min) Zinc (2, 4 min) | [25] |
| Brown rice | HPP (100, 300, 500 MPa for 10 min) | Surface non-uniform, with cavities | ↓ Iron (100, 500 MPa) ↓ Zinc (100–500 MPa) ↓ Copper (100, 300 MPa) | No increases | Iron | [69] |
| Milk-based fruit beverages | HPP (400 MPa for 5 min) | No information provided about structure | ↓ Calcium | No increases | No decreases | [71] |
| Mango and papaya juice sweetened with Stevia rebaudiana | Pulsed electric fields (PEF) (32 and 256 kJ/kg) Ultrasounds (US) (32 and 256 kJ/kg) | No information provided about structure | ↓ Vitamin C | No bioaccessible | No bioaccessible | [42] |
| Fruit juice milk-based beverage | HPP (400 MPa for 5 min) | No information provided about structure | ↓ α-tocopherol (HPP + whole and skimmed milk), γ- tocopherol (HPP + whole milk) ↑ α-tocopherol, γ- tocopherol, δ-tocopherol (HPP + soymilk) ↓ Vitamin C (HPP + skimmed milk and soymilk) | No increases | α-tocopherol, γ- tocopherol, δ-tocopherol (HPP + soymilk) Vitamin C (HPP + whole milk or soymilk) | [47] |
| Gazpacho (vegetable soup) | PEF (35 kV/cm for 750 µs) | No information provided about structure | No changes | No increases | No decreases | [73] |
| Orange juice | PEF (35 kV/cm for 750 µs) | No information provided about structure | No changes | No increases | No decreases | [72] |
| Fruit juice-based beverage mixed with soymilk | PEF (35 kV/cm for 1800 μs) HPP (400 MPa for 5 min) | No information provided about structure | ↓ Vitamin C (PEF and HPP) | Vitamin C (HPP) | No decreases | [63] |
| Cashew apple bagasse puree | US (500 W for 2, 6, 10 min) | Microchannels and cell disruption | ↑ Vitamin C | Vitamin C | No decreases | [74] |
4. Concluding Remarks and Further Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rodríguez-Roque, M.J.; Rojas-Graü, M.A.; Elez-Martínez, P.; Martín-Belloso, O. In vitro bioaccessibility of health-related compounds from a blended fruit juice-soymilk beverage: Influence of the food matrix. J. Funct. Foods 2014, 7, 161–169. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrì, F.; Boutrou, R.; Corredig, F.M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. Food Funct 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribas-Agustí, A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Elez-Martínez, P. Food processing strategies to enhance phenolic compounds bioaccessibility and bioavailability in plant-based foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2531–2548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barba, F.J.; Mariutti, L.R.B.; Bragagnolo, N.; Mercadante, A.Z.; Barbosa-Cánovas, G.V.; Orlien, V. Bioaccessibility of bioactive compounds from fruits and vegetables after thermal and nonthermal processing. Trends Food Sci. Technol. 2017, 67, 195–206. [Google Scholar] [CrossRef]
- Failla, M.L.; Chitchumroonchokchai, C.; Ishida, B.K. In vitro micellarization and intestinal cell uptake of cis isomers of lycopene exceed those of all-trans lycopene. J. Nutr. 2008, 138, 482–486. [Google Scholar] [CrossRef]
- Nagarajan, J.; Ramanan, R.N.; Raghunandan, M.E.; Galanakis, C.M.; Krishnamurthy, N.P. Carotenoids; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128052570. [Google Scholar]
- Failla, M.L.; Chitchumroonchokchai, C. In vitro models as tools for screening the relative bioavailabilities of provitamin A carotenoids in foods. Harvest Plus Tech. Monogr. 2005, 3, 32. [Google Scholar]
- Granado-Lorencio, F.; Olmedilla-Alonso, B.; Herrero-Barbudo, C.; Pérez-Sacristán, B.; Blanco-Navarro, I.; Blázquez-García, S. Comparative in vitro bioaccessibility of carotenoids from relevant contributors to carotenoid intake. J. Agric. Food Chem. 2007, 55, 6387–6394. [Google Scholar] [CrossRef] [PubMed]
- Sy, C.; Gleize, B.; Dangles, O.; Landrier, J.-F.; Veyrat, C.C.; Borel, P. Effects of physicochemical properties of carotenoids on their bioaccessibility, intestinal cell uptake, and blood. Mol. Nutr. Food Res. 2012, 56, 1385–1397. [Google Scholar] [CrossRef] [PubMed]
- Tyssandier, V.; Lyan, B.; Borel, P. Main factors governing the transfer of carotenoids from emulsion lipid droplets to micelles. Biochim. Biophys. Acta 2001, 1533, 285–292. [Google Scholar] [CrossRef]
- Mapelli-Brahm, P.; Corte-Real, J.; Meléndez-Martínez, A.J.; Bohn, T. Bioaccessibility of phytoene and phytofluene is superior to other carotenoids from selected fruit and vegetable juices. Food Chem. 2017, 229, 304–311. [Google Scholar] [CrossRef]
- Schweiggert, R.M.; Mezger, D.; Schimpf, F.; Steingass, C.B.; Carle, R. Influence of chromoplast morphology on carotenoid bioaccessibility of carrot, mango, papaya, and tomato. Food Chem. 2012, 135, 2736–2742. [Google Scholar] [CrossRef]
- Panozzo, A.; Lemmens, L.; Van Loey, A.; Manzocco, L.; Nicoli, M.C.; Hendrickx, M. Microstructure and bioaccessibility of different carotenoid species as affected by high pressure homogenisation: A case study on differently coloured tomatoes. Food Chem. 2013, 141, 4094–4100. [Google Scholar] [CrossRef] [PubMed]
- Palmero, P.; Lemmens, L.; Ribas-Agustí, A.; Sosa, C.; Met, K.; De Dieu Umutoni, J.; Hendrickx, M.; Van Loey, A. Novel targeted approach to better understand how natural structural barriers govern carotenoid in vitro bioaccessibility in vegetable-based systems. Food Chem. 2013, 141, 2036–2043. [Google Scholar] [CrossRef]
- Jeffery, J.L.; Turner, N.D.; King, S.R. Carotenoid bioaccessibility from nine raw carotenoid-storing fruits and vegetables using an in vitro model. J. Sci. Food Agric. 2012, 92, 2603–2610. [Google Scholar] [CrossRef]
- Jeffery, J.; Holzenburg, A.; King, S. Physical barriers to carotenoid bioaccessibility. Ultrastructure survey of chromoplast and cell wall morphology in nine carotenoid-containing fruits and vegetables. J. Sci. Food Agric. 2012, 92, 2594–2602. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J. Food Sci. 2011, 76, 6–15. [Google Scholar] [CrossRef] [Green Version]
- Cilla, A.; Bosch, L.; Barberá, R.; Alegría, A. Effect of processing on the bioaccessibility of bioactive compounds—A review focusing on carotenoids, minerals, ascorbic acid, tocopherols and polyphenols. J. Food Compos. Anal. 2018, 68, 3–15. [Google Scholar] [CrossRef]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Jakobek, L.; Mati, P. Non-covalent dietary fiber—Polyphenol interactions and their influence on polyphenol bioaccessibility. Trends Food Sci. Technol. 2019, 83, 235–247. [Google Scholar] [CrossRef]
- Oliveira, A.; Amaro, A.L.; Pintado, M. Impact of food matrix components on nutritional and functional properties of fruit-based products. Curr. Opin. Food Sci. 2018, 22, 153–159. [Google Scholar] [CrossRef]
- Thakur, N.; Raigond, P.; Singh, Y.; Mishra, T.; Singh, B.; Lal, M.K.; Dutt, S. Recent updates on bioaccessibility of phytonutrients. Trends Food Sci. Technol. 2020, 97, 366–380. [Google Scholar] [CrossRef]
- Drago, S.R. Minerals. In Nutraceutical and Functional Food Components; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 129–157. ISBN 9780128052570. [Google Scholar]
- Briones-Labarca, V.; Venegas-Cubillos, G.; Ortiz-Portilla, S.; Chacana-Ojeda, M.; Maureira, H. Effects of high hydrostatic pressure (HHP) on bioaccessibility, as well as antioxidant activity, mineral and starch contents in Granny Smith apple. Food Chem. 2011, 128, 520–529. [Google Scholar] [CrossRef]
- Cilla, A.; Barberá, R.; López-García, G.; Blanco-Morales, V.; Alegría, A.; Garcia-Llatas, G. Impact of Processing on Mineral Bioaccessibility/Bioavailability; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128141748. [Google Scholar]
- Khaneghah, A.M.; Bagher Hashemi, S.M.; Es, I.; Gholamhosseinpour, A.; Loizzo, M.R.; Giardinieri, A.; Pacetti, D.; Pourmohammadi, K.; Ferreira, D.S. Water-Soluble Vitamins; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128141748. [Google Scholar]
- Gironés-Vilaplana, A.; Villaño, D.; Marhuenda, J.; Moreno, D.A.; García-Viguera, C. Vitamins. In Nutraceutical and Functional Food Components; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 159–201. ISBN 9780128052570. [Google Scholar]
- Leong, S.Y.; Du, D.; Oey, I. Pulsed Electric Fields enhances calcium infusion for improving the hardness of blanched carrots. Innov. Food Sci. Emerg. Technol. 2018, 47, 46–55. [Google Scholar] [CrossRef]
- López-Gámez, G.; Elez-Martínez, P.; Quiles-Chuliá, A.; Martín-Belloso, O.; Hernando-Hernando, I.; Soliva-Fortuny, R. Effect of pulsed electric fields on carotenoid and phenolic bioaccessibility and their relationship with carrot structure. Food Funct. 2021. [Google Scholar] [CrossRef]
- González-Casado, S.; Martín-Belloso, O.; Elez-Martínez, P.; Soliva-Fortuny, R. Application of pulsed electric fields to tomato fruit for enhancing the bioaccessibility of carotenoids in derived products. Food Funct. 2018, 9, 2282–2289. [Google Scholar] [CrossRef] [Green Version]
- Bot, F.; Verkerk, R.; Mastwijk, H.; Anese, M.; Fogliano, V.; Capuano, E. The effect of pulsed electric fields on carotenoids bioaccessibility: The role of tomato matrix. Food Chem. 2018, 240, 415–421. [Google Scholar] [CrossRef]
- Jayathunge, K.G.L.R.; Stratakos, A.C.; Cregenzán-Albertia, O.; Grant, I.R.; Lyng, J.; Koidis, A. Enhancing the lycopene in vitro bioaccessibility of tomato juice synergistically applying thermal and non-thermal processing technologies. Food Chem. 2017, 221, 698–705. [Google Scholar] [CrossRef] [Green Version]
- Sentandreu, E.; Stinco, C.M.; Vicario, I.M.; Mapelli-Brahm, P.; Navarro, J.L.; Meléndez-Martínez, A.J. High-pressure homogenization as compared to pasteurization as a sustainable approach to obtain mandarin juices with improved bioaccessibility of carotenoids and flavonoids. J. Clean. Prod. 2020, 262. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, Y.; Xie, F.; Gu, X.; Wu, J.; Wang, Z. High pressure homogenization versus ultrasound treatment of tomato juice: Effects on stability and in vitro bioaccessibility of carotenoids. Lwt 2019, 116, 108597. [Google Scholar] [CrossRef]
- Anese, M.; Bot, F.; Panozzo, A.; Mirolo, G.; Lippe, G. Effect of ultrasound treatment, oil addition and storage time on lycopene stability and in vitro bioaccessibility of tomato pulp. Food Chem. 2015, 172, 685–691. [Google Scholar] [CrossRef]
- Anese, M.; Mirolo, G.; Beraldo, P.; Lippe, G. Effect of ultrasound treatments of tomato pulp on microstructure and lycopene in vitro bioaccessibility. Food Chem. 2013, 136, 458–463. [Google Scholar] [CrossRef]
- Mercado-Mercado, G.; Montalvo-González, E.; González-Aguilar, G.A.; Alvarez-Parrilla, E.; Sáyago-Ayerdi, S.G. Ultrasound-assisted extraction of carotenoids from mango (Mangifera indica L. ‘Ataulfo’) by-products on in vitro bioaccessibility. Food Biosci. 2018, 21, 125–131. [Google Scholar] [CrossRef]
- Cano, M.P.; Gómez-Maqueo, A.; Fernández-López, R.; Welti-Chanes, J.; García-Cayuela, T. Impact of high hydrostatic pressure and thermal treatment on the stability and bioaccessibility of carotenoid and carotenoid esters in astringent persimmon (Diospyros kaki Thunb, var. Rojo Brillante). Food Res. Int. 2019, 123, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Moelants, K.R.N.; Lemmens, L.; Vandebroeck, M.; Van Buggenhout, S.; Van Loey, A.M.; Hendrickx, M.E. Relation between particle size and carotenoid bioaccessibility in carrot- and tomato-derived suspensions. J. Agric. Food Chem. 2012, 60, 11995–12003. [Google Scholar] [CrossRef] [PubMed]
- Knockaert, G.; Pulissery, S.K.; Colle, I.; Van Buggenhout, S.; Hendrickx, M.; Loey, A. Van Lycopene degradation, isomerization and in vitro bioaccessibility in high pressure homogenized tomato puree containing oil: Effect of additional thermal and high pressure processing. Food Chem. 2012, 135, 1290–1297. [Google Scholar] [CrossRef]
- Buniowska, M.; Carbonell-Capella, J.M.; Frigola, A.; Esteve, M.J. Bioaccessibility of bioactive compounds after non-thermal processing of an exotic fruit juice blend sweetened with Stevia rebaudiana. Food Chem. 2017, 221, 1834–1842. [Google Scholar] [CrossRef]
- Colle, I.; Van Buggenhout, S.; Van Loey, A.; Hendrickx, M. High pressure homogenization followed by thermal processing of tomato pulp: Influence on microstructure and lycopene in vitro bioaccessibility. Food Res. Int. 2010, 43, 2193–2200. [Google Scholar] [CrossRef]
- Knockaert, G.; Lemmens, L.; Van Buggenhout, S.; Hendrickx, M.; Van Loey, A. Changes in β-carotene bioaccessibility and concentration during processing of carrot puree. Food Chem. 2012, 133, 60–67. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Bi, J.; Yi, J.; Peng, J.; Ning, C.; Wellala, C.K.D.; Zhang, B. Effects of high pressure homogenization on pectin structural characteristics and carotenoid bioaccessibility of carrot juice. Carbohydr. Polym. 2019, 203, 176–184. [Google Scholar] [CrossRef] [PubMed]
- de Souza Carvalho, L.M.; Lemos, M.C.M.; Sanches, E.A.; da Silva, L.S.; de Araújo Bezerra, J.; Aguiar, J.P.L.; das Chagas do Amaral Souza, F.; Alves Filho, E.G.; Campelo, P.H. Improvement of the bioaccessibility of bioactive compounds from Amazon fruits treated using high energy ultrasound. Ultrason. Sonochem. 2020, 67, 105148. [Google Scholar] [CrossRef]
- Cilla, A.; Alegría, A.; de Ancos, B.; Sánchez-Moreno, C.; Cano, M.P.; Plaza, L.; Clemente, G.; Lagarda, M.J.; Barberá, R. Bioaccessibility of tocopherols, carotenoids, and ascorbic acid from milk- and soy-based fruit beverages: Influence of food matrix and processing. J. Agric. Food Chem. 2012, 60, 7282–7290. [Google Scholar] [CrossRef]
- Zhong, S.; Vendrell-Pacheco, M.; Heskitt, B.; Chitchumroonchokchai, C.; Failla, M.; Sastry, S.K.; Francis, D.M.; Martin-Belloso, O.; Elez-Martínez, P.; Kopec, R.E. Novel Processing Technologies as Compared to Thermal Treatment on the Bioaccessibility and Caco-2 Cell Uptake of Carotenoids from Tomato and Kale-Based Juices. J. Agric. Food Chem. 2019, 67, 10185–10194. [Google Scholar] [CrossRef]
- Stinco, C.M.; Sentandreu, E.; Mapelli-Brahm, P.; Navarro, J.L.; Vicario, I.M.; Meléndez-Martínez, A.J. Influence of high pressure homogenization and pasteurization on the in vitro bioaccessibility of carotenoids and flavonoids in orange juice. Food Chem. 2020, 331, 127259. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Paulino, M.; Stinco, C.M.; Mapelli-Brahm, P.; Wang, X.D. Study of the time-course of cis/trans (Z/E) isomerization of lycopene, phytoene, and phytofluene from tomato. J. Agric. Food Chem. 2014, 62, 12399–12406. [Google Scholar] [CrossRef]
- Cervantes-Paz, B.; de Jesús Ornelas Paz, J.; Pérez-Martínez, J.D.; Reyes-Hernández, J.; Zamudio-flores, P.B.; Rios-velasco, C.; Ibarra-Junquera, V.; Ruiz-Cruz, S. Effect of pectin concentration and properties on digestive events involved on micellarization of free and esterified carotenoids. Food Hydrocoll. 2016, 60, 580–588. [Google Scholar] [CrossRef]
- Gence, L.; Servent, A.; Poucheret, P.; Hiol, A.; Dhuique-Mayer, C. Pectin structure and particle size modify carotenoid bioaccessibility and uptake by Caco-2 cells in citrus juices: Vs. concentrates. Food Funct. 2018, 9, 3523–3531. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, L.; Van Buggenhout, S.; Van Loey, A.M.; Hendrickx, M.E. Particle size reduction leading to cell wall rupture is more important for the β-carotene bioaccessibility of raw compared to thermally processed carrots. J. Agric. Food Chem. 2010, 58, 12769–12776. [Google Scholar] [CrossRef] [PubMed]
- Faulks, R.M.; Southon, S. Challenges to understanding and measuring carotenoid bioavailability. Biochim. Biophys. Acta 2005, 1740, 95–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, O.E.; Pilosof, A.M.R. Pulsed electric fields effects on the molecular structure and gelation of β -lactoglobulin concentrate and egg white. Food Res. Int. 2004, 37, 102–110. [Google Scholar] [CrossRef]
- Palmero, P.; Lemmens, L.; Hendrickx, M.; Loey, A. Van Role of carotenoid type on the effect of thermal processing on bioaccessibility. Food Chem. 2014, 157, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Huo, T.; Ferruzzi, M.G.; Schwartz, S.J.; Failla, M.L. Impact of fatty acyl composition and quantity of triglycerides on bioaccessibility of dietary carotenoids. J. Agric. Food Chem. 2007, 55, 8950–8957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribas-Agustí, A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Elez-Martínez, P. Influence of pulsed electric fields processing on the bioaccessible and non-bioaccessible fractions of apple phenolic compounds. J. Funct. Foods 2019, 59, 206–214. [Google Scholar] [CrossRef]
- He, Z.; Tao, Y.; Zeng, M.; Zhang, S.; Tao, G.; Qin, F.; Chen, J. High pressure homogenization processing, thermal treatment and milk matrix affect in vitro bioaccessibility of phenolics in apple, grape and orange juice to different extents. Food Chem. 2016, 200, 107–116. [Google Scholar] [CrossRef]
- Zudaire, L.; Lafarga, T.; Viñas, I.; Abadias, M.; Brunton, N.; Aguiló-Aguayo, I. Effect of Ultrasound Pre-Treatment on the Physical, Microbiological, and Antioxidant Properties of Calçots. Food Bioprocess Technol. 2019, 12, 387–394. [Google Scholar] [CrossRef] [Green Version]
- Lafarga, T.; Rodríguez-Roque, M.J.; Bobo, G.; Villaró, S.; Aguiló-Aguayo, I. Effect of ultrasound processing on the bioaccessibility of phenolic compounds and antioxidant capacity of selected vegetables. Food Sci. Biotechnol. 2019, 28, 1713–1721. [Google Scholar] [CrossRef]
- de Oliveira Ribeiro, L.; Braga Pinheiro, A.C.; Santa Brígida, A.I.; Asenova Genisheva, Z.; Martins de Oliveira Soares Vicente, A.A.; Couto Teixeira, J.A.; Martins de Matta, V.; Pereira Freitas, S. In vitro gastrointestinal evaluation of a juçara-based smoothie: Effect of processing on phenolic compounds bioaccessibility. J. Food Sci. Technol. 2019, 56, 5017–5026. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Roque, M.J.; Ancos, B.; Sánchez-Moreno, C.; Cano, M.P.; Elez-Martínez, P.; Martín-Belloso, O. Impact of food matrix and processing on the in vitro bioaccessibility of vitamin C, phenolic compounds, and hydrophilic antioxidant activity from fruit juice-based beverages. J. Funct. Foods 2015, 14, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Roque, M.J.; De Ancos, B.; Sánchez-Vega, R.; Sánchez-Moreno, C.; Elez-Martínez, P.; Martín-Belloso, O. In vitro bioaccessibility of isoflavones from a soymilk-based beverage as affected by thermal and non-thermal processing. Innov. Food Sci. Emerg. Technol. 2020, 66, 102504. [Google Scholar] [CrossRef]
- Quan, W.; Tao, Y.; Qie, X.; Zeng, M.; Qin, F.; Chen, J.; He, Z. Effects of high-pressure homogenization, thermal processing, and milk matrix on the in vitro bioaccessibility of phenolic compounds in pomelo and kiwi juices. J. Funct. Foods 2020, 64, 103633. [Google Scholar] [CrossRef]
- Alminger, M.; Aura, A.M.; Bohn, T.; Dufour, C.; El, S.N.; Gomes, A.; Karakaya, S.; Martínez-Cuesta, M.C.; Mcdougall, G.J.; Requena, T.; et al. In vitro models for studying secondary plant metabolite digestion and bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2014, 13, 413–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Aguilar, G.A.; Blancas-Benítez, F.J.; Sáyago-Ayerdi, S.G. Polyphenols associated with dietary fibers in plant foods: Molecular interactions and bioaccessibility. Curr. Opin. Food Sci. 2017, 13, 84–88. [Google Scholar] [CrossRef]
- Mercado-Mercado, G.; Blancas-Benitez, F.J.; Velderrain-Rodríguez, G.R.; Montalvo-González, E.; González-Aguilar, G.A.; Alvarez-Parrilla, E.; Sáyago-Ayerdi, S.G. Bioaccessibility of polyphenols released and associated to dietary fibre in calyces and decoction residues of Roselle (Hibiscus sabdariffa L.). J. Funct. Foods 2015, 18, 171–181. [Google Scholar] [CrossRef]
- Xia, Q.; Wang, L.; Xu, C.; Mei, J.; Li, Y. Effects of germination and high hydrostatic pressure processing on mineral elements, amino acids and antioxidants in vitro bioaccessibility, as well as starch digestibility in brown rice (Oryza sativa L.). Food Chem. 2016, 214, 533–542. [Google Scholar] [CrossRef]
- Briones-Labarca, V.; Muñoz, C.; Maureira, H. Effect of high hydrostatic pressure on antioxidant capacity, mineral and starch bioaccessibility of a non conventional food: Prosopis chilensis seed. FRIN 2011, 44, 875–883. [Google Scholar] [CrossRef]
- Cilla, A.; Lagarda, M.J.; Alegría, A.; De Ancos, B.; Cano, M.P.; Sánchez-moreno, C.; Plaza, L.; Barberá, R. Effect of processing and food matrix on calcium and phosphorous bioavailability from milk-based fruit beverages in Caco-2 cells. FRIN 2011, 44, 3030–3038. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Moreno, C.; Cano, P.; de Ancos, B.; Plaza, L.; Olmedilla, B.; Granado, F.; Elez-Martínez, P.; Martín-Belloso, O.; Martín, A. Pulsed electric fields – processed orange juice consumption increases plasma vitamin C and decreases F2-isoprostanes in healthy humans. J. Nutr. Biochem. 2004, 15, 601–607. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Cano, M.P.; de Ancos, B.; Plaza, L.; Olmedilla, B.; Granado, F.; Elez-Martínez, P.; Martín-Belloso, O.; Martín, A. Intake of Mediterranean vegetable soup treated by pulsed electric fields affects plasma vitamin C and antioxidant biomarkers in humans. Int. J. Food Sci. Nutr. 2005, 56, 115–124. [Google Scholar] [CrossRef]
- Fonteles, T.V.; Leite, A.K.F.; Silva, A.R.A.; Carneiro, A.P.G.; Miguel, E.D.C.; Cavada, B.S.; Fernandes, F.A.N.; Rodrigues, S. Ultrasound processing to enhance drying of cashew apple bagasse puree: Influence on antioxidant properties and in vitro bioaccessibility of bioactive compounds. Ultrason. Sonochem. 2016, 31, 237–249. [Google Scholar] [CrossRef]


| Food Matrix | Processing Conditions | Structure | Carotenoid Content | Bioaccessibility Increase | Bioaccessibility Decrease | References | |
|---|---|---|---|---|---|---|---|
| Carrot | Pulsed electric fields (PEF) (0.9 and 191 kJ/kg) in water PEF (0.9 and 191 kJ/kg) in 300 ppm CaCl2 PEF + Blanching (B) (100 °C for 5 min) | PEF (191 kJ/kg in water or in 300 ppm CaCl2): Decrease hardness PEF (191 kJ/kg in CaCl2 + B): Increase hardness | No changes in β-carotene content | No changes | No changes | [29] | |
| Carrot | PEF (five pulses of 3.5 kV/cm) | ↓ Firmness Degradation of cell walls Changes in carotenoid location | No changes in carotenoid content | Total carotenoids, α-carotene, β-carotene | No decreases | [30] | |
| Tomato puree (5% olive oil) | PEF (0.02–2.31 kJ/kg) applied to whole tomato | ↓ Firmness (0.06–2.31 kJ/kg) | ↑ Total (0.06–2.31 kJ/kg) ↑ β-carotene (0.06–0.38 kJ/kg; 1.38–2.31 kJ/kg) ↑ Lycopene (0.14–2.31 kJ/kg) ↑ Lutein (0.14, 0.5–2.31 kJ/kg) ↑ Phytofluene, phytoene, δ-carotene ↑ ɣ-carotene (0.09, 0.14, 2.31 kJ/kg) | Total (0.38 kJ/kg), β-carotene (0.38 kJ/kg), lycopene (0.09–0.38 kJ/kg; 1.38–2.31 kJ/kg), lutein (0.09–0.38 kJ/kg), ɣ-carotene (0.09–0.38; 0.83–2.31 kJ/kg) | Total (0.02 and 0.5 kJ/kg), β-carotene (0.02 and 0.06 kJ/kg), lycopene (0.02 and 0.06 kJ/kg), lutein (0.06 and 0.5 kJ/kg), phytofluene (0.02, 0.06, 0.14, 0.5, 0.83, 1.38 and 2.31 kJ/kg), phytoene, δ-carotene (0.02–0.06 kJ/kg; 0.5–2.31 kJ/kg) | [31] | |
| Tomato fractions | PEF (7.6 MJ/kg; 40–45 °C) PEF + Heat (H) (7.6 MJ/kg; 85–90 °C) | Tissue | PEF + H: cell detachment | No changes | No increases | all-trans-lycopene (H; PEF + H) | [32] |
| Cell clusters | Cell membranes damaged | ↓ β-carotene (PEF; H) | No changes | ||||
| Single cells | Cell membranes damaged | ↓ β-carotene (PEF; H) | No changes | ||||
| Chromoplasts | No differences | ↓ β-carotene (PEF; H; PEF + H) and all-trans-lycopene (PEF; PEF + H) | all-trans-lycopene and β-carotene (PEF; PEF + H) | ||||
| Tomato | PEF (1 kV/cm for 0, 4, 80 or 320 μs) Storage: 0 h, 24 h or 48 h | Irregular cell wall structure by increasing holding time treatment | ↑ Total lycopene (all treatments) ↑ All-trans-lycopene (80 and 320 μs) ↑ Cis-lycopene (all treatments excepting 4 μs at 0 h) | Total lycopene (4 μs at 24 h) All-trans-lycopene (4 μs at 0 h) Cis-lycopene (all treatments at 24 h and 320 μs at 0 h) | Total lycopene (80 μs; 320 μs at 24 and 48 h) All-trans-lycopene (80 and 320 μs at 0 and 24 h and 320 μs at 48 h) Cis-lycopene (all treatments at 48 h and 4 μs at 0 h) | [33] | |
| Tomato juice | PEF (1 kV/cm for 4 μs) B (90 °C for 2 min) PEF + B PEF + B + PEF2 (35 kV/cm for 1500 μs) PEF + B + Ultrasounds (US) (20 kHz; 20% amplitude; 7 min) PEF + B + US + PEF2 | No information provided about structure | ↑ Total lycopene (all treatments) ↑ All-trans-lycopene (PEF, PEF + B + US, PEF + B + PEF2) ↑ Cis-lycopene (PEF, PEF + B + PEF2) ↓ Cis-lycopene (PEF + B + US, PEF + B + US + PEF2) | Cis-lycopene (all treatments) Trans-lycopene (PEF + B + PEF2, PEF + B + US + PEF2) | Trans-lycopene (B, B + US) | [33] | |
| Mandarin juices | High pressure homogenization (HPH) (150 MPa reaching 68 °C for 15 s) | Cell rupture and ↓ particle size | ↓ Total carotenoids and individual (lutein, zeaxanthin, zeinoxanthin, β-carotene, α-carotene, β-cryptoxanthin, phytofluene, phytoene, cis-violaxanthin isomers, 9-cis- violaxanthin + cis-antheraxanthin isomers, cis-anteraxanthin isomers, luteoxanthin isomers, mutatoxanthin isomers) | Total carotenoids and individual (lutein, zeaxanthin, zeinoxanthin, β-carotene, α-carotene, β-cryptoxanthin, phytofluene, phytoene, cis-violaxanthin isomers, 9-cis-violaxanthin + cis-antheraxanthin isomers, cis- anteraxanthin isomers, luteoxanthin isomers, mutatoxanthin isomers) | No decreases | [34] | |
| Tomato juice | HPH (200, 300, 400, and 500 bar) (2 cycles of 15 min) | ↓ particle size by increasing pressure | ↑ All-trans-lycopene (200 bar) ↓ All-trans-lycopene (300–500 bar) ↓ 5-cis-lycopene (400 and 500 bar) ↑ 5-cis-lycopene (200 and 300 bar) ↓ 9-cis-lycopene (all treatments) ↑ 13-cis-lycopene (300 and 400 bar) ↓ 13-cis-lycopene (200 and 500 bar) ↑ β-carotene (200 bar) | All-trans-lycopene and isomers (500 bar) | All-trans-lycopene and total lycopene (200 bar) | [35] | |
| Tomato juice | US (25 Hz; 200 W, 400 W, 600 W and 800 W for 20 min) | Similar increase in particle size in all treatments | ↑ All-trans-lycopene (200 and 400 W) ↓ All-trans-lycopene (600 W) ↓ 5-cis-lycopene (all treatments) ↑ 9-cis-lycopene (200, 400, 800 W) ↑ 13-cis-lycopene, ζ-carotene (all treatments) | All-trans-lycopene and isomers (800 W) | All carotenoids (400 W) | [35] | |
| Commercial pasteurized tomato pulp (with or without added sunflower oil) | US (30 min; 24 kHz; 100 μm; 71 W; 1462 J/cm3) | US-treated samples showed broken cells with lycopene distributed in the matrix | No changes in lycopene content | Lycopene (in US-treated samples with 5% of oil) | No decreases in lycopene (in US-treated samples with 0, 2.5 and 10% of oil) | [36] | |
| Commercial pasteurized tomato pulp | US (15, 30 and 60 min; 100 μm; 105 W/cm2) | Loss of cell integrity when increasing time of treatments. No intact cells after 60 min. Decrease in pectin esterification degree and increase in viscosity | No changes in lycopene content | No increases | Lycopene bioaccessibility decreases by increasing processing time | [37] | |
| Mango by-products (peel and paste) | US (30 min; 30% of amplitude; 9 W/mL) | No information available about structural characteristics | ↑ β-cryptoxanthin (in peel and paste), lutein and β-carotene (both ↓ in paste and ↑ in peel) Content was determined in gastric phase | Total carotenoids, β-cryptoxanthin, lutein and β-carotene (in peel and paste) | No decreases | [38] | |
| Astringent persimmon | High pressure processing (HPP) (200 MPa for 6 min) | No information provided about structure | ↓ Total carotenoids and xanthophyll esters, lycopene, (all-trans)-lutein 3-O-laurate-3′-O- Myristate, -β-cryptoxanthin myristate, -antheraxanthin myristate-Palmitate, -zeaxanthin myristate, -antheraxanthin 3-O-Palmitate, -lutein 3-O-palmitate, -lutein dimyristate, -antheraxanthin laurate-myristate, -α-carotene, -α-cryptoxanthin, -violaxanthin, -antheraxanthin, -neoxanthin dibutyrate, -violaxanthin palmitate, -violaxanthin laurate, 9-cis neoxanthin dibutyrate and 9-cis-β-carotene ↑ (all-trans)-zeaxanthin, -β-cryptoxanthin, -β-carotene and 9-cis-α-carotene | (All-trans)-anteraxanthin, -lutein, -zeaxantin, -β-cryptoxanthin, (all-trans and 13-cis) -α-, -β-carotene, (all-trans)-violaxanthin laurate, (all-trans)-zeaxanthin palmitate, (all-trans)-β-cryptoxanthin laurate, (all-trans)-lutein 3-O-palmitate, (all-trans)-zeaxanthin myristate, (all-trans)-antheraxanthin myristate-palmitate, (all-trans)-β-cryptoxanthin myristate, (all-trans)-β-cryptoxanthin dipalmitate and lycopene | No decreases | [39] | |
| Carrot and tomato purees | HPH at 20 MPa Blended carrot/tomato purees Homogenized carrot/tomato purees During digestion: no addition of oil, addition of olive oil (2%) or oil emulsion (2%) | Different suspensions with particle size were prepared through wet sieving technique. The cell wall of particles smaller than 125 μm was damaged. | No information provided about carotenoid content before digestion | Carrot puree without oil: All-trans-β-carotene (≤125 μm) Carrot puree with 2% oil: All-trans-β-carotene (≤125 μm) Carrot puree with 2% oil emulsion: All-trans-β-carotene (≤125 μm) Tomato puree 2% oil emulsion: all-trans-lycopene (<40 μm HPH) | No decreases | [40] | |
| Tomato puree (5% olive oil) | High pressure pasteurization (HP-P) (HPP 450 MPa for 15 min and 20 °C and 600 MPa for 20 min and 45 °C) High pressure sterilization (HP-S) (121.1 °C for 1.5 min and 117 °C for 3 min at 600 MPa) | Particle size was the same among treatments | ↑ 13-cis-lycopene (HP-S), 9-cis-lycopene (HP-S, 3 min), 5-cis-lycopene (HP-P, HP-S, 3 min) ↓ lycopene, all-trans-lycopene, (HP-S) | No increases | Lycopene in HP-S | [41] | |
| Mango and papaya juice sweetened with Stevia rebaudiana | PEF (32 and 256 kJ/kg) US (32 and 256 kJ/kg) | No information provided about structure | PEF (32 kJ/kg): ↑ Total carotenoids US (32 and 256 kJ/kg): ↓ Total carotenoids | PEF (32 and 256 kJ/kg) and US (32 kJ/kg): Total carotenoids | No decreases | [42] | |
| Tomato pulp | HPH (84–1327 bar) HPH (220, 521, 1135 bar) + H (30 min, 90 °C) | ↑ Homogenization pressure resulted in the breakdown of the tomato cell aggregate structures and volumetric percentage of the small particles increased ↑ Strength of the fiber network | No changes | No increases | Decrease in lycopene by increasing pressure up to 479 MPa. After that, it remained constant | [43] | |
| Carrot puree without oil and adding 5% olive oil | HPH (10, 50 or 100 MPa for 1 cycle) HPH (100 MPa) + HP-P (20 min at 600 MPa and 45 °C) | ↓ Particle size by increasing pressure | Carrot puree (HPH): ↑ 13-cis- β-carotene Carrot puree (5% olive oil) (HPH): No changes Carrot puree (HPH + HP-P): ↓ All-trans- β-Carotene and total β-Carotene Carrot puree (5% olive oil) (HPH + HP-P): ↑ 9-cis- β -Carotene and total β-Carotene | Carrot puree and puree with added oil (HPH): β-carotene (50 MPa and 100 MPa) Carrot puree (HPH + HP-P): No changes compared to untreated, but higher bioaccessibility than just HPH treated purees | No decreases | [44] | |
| Tomato pulps (red, orange, and yellow) | HPH (single pass at 20, 50 and 100 MPa) | Consistency increase by increasing pressure. ↓ particle dimensions Single cells and broken material (20 MPa) Cell fragments (50 MPa) Complete breakage of cells (100 MPa) | Red: Lycopene and lutein decrease by increasing pressure Orange: ↓ ζ-carotene by increasing pressure Yellow: ↓ Lutein | No increases | All carotenoids decreased in all treatments | [13] | |
| Carrot juice | HPH (20 MPa, 60 MPa, 100 MPa, 150 MPa and 180 MPa) (fixed 1 pass at 25 °C) Pass of 1, 2 and 3 (fixed 60 MPa at 25 °C) Inlet temperature of 25 °C, 50 °C and 70 °C (fixed 60 MPa and 1 pass) | No information provided about structure | ↑ Total carotenoids (180 MPa) | Total carotenoids after each pressure treatment Total carotenoids after 3 passes Total carotenoids at 50 and 70 °C | No decreases | [45] | |
| Buriti juice | US (0, 0.9, 1.8, 2.7 and 3.6 kJ/cm3) | No information provided about structure | ↑ β-carotene after all treatments | β-carotene after all treatments | No decreases | [46] | |
| Fruit juice milk-based beverage | HPP (400 MPa for 5 min) | No information provided about structure | ↑ Total carotenoids, neoxanthin, 9-cis-violaxanthin (HPP + whole milk), zeaxanthin, lutein (HPP + whole milk or skimmed milk), ↓ Total carotenoids (HPP + soymilk) Zeaxanthin (HPP + soymilk), lutein | Total carotenoids (HPP + soymilk) neoxanthin, 9-cis-violaxanthin (HPP + whole milk or soymilk) zeaxanthin, lutein (HPP + soymilk) | Total carotenoids (HPP + whole milk) Zeaxanthin, lutein (HPP + whole milk or skimmed milk) | [47] | |
| Tomato juice and kale-based juice | PEF (35 kV/cm for 1000 µs) HPP (500 MPa for 3 min) | No information provided about structure | ↓ β-carotene and lutein (PEF-treated kale-based juice) | Lycopene (PEF-treated tomato juice) | β-carotene (PEF-treated tomato juice) | [48] | |
| Orange juice | HPH (150 MPa reaching 68 °C for 15 s) | No information provided about structure | ↓ Total carotenoids,antheraxanthin, violaxanthin, luteoxanthin, zeaxanthin, antheraxanthin, β-cryptoxanthin, α-carotene, β-carotene, phytoene | Lutein, zeaxanthin, zeinoxanthin, β-cryptoxanthin, α-carotene, β-carotene, phytoene, phytofluene, violaxanthin, antheraxanthin, luteoxanthin, mutatoxanthin | No decreases | [49] | |
| Food Matrix | Processing Conditions | Structure | Phenolic Content | Bioaccessibility Increase | Bioaccessibility Decrease | References | |
|---|---|---|---|---|---|---|---|
| Mango and papaya juice sweetened with Stevia rebaudiana | Pulsed electric fields (PEF) (32 and 256 kJ/kg) Ultrasounds (US) (32 and 256 kJ/kg) | No information provided about structure | PEF (32 kJ/kg): ↑ Total phenolic content US (32 kJ/kg): ↓ Total anthocyanins | PEF (256 kJ/kg) and US (32 and 256 kJ/kg): Total phenolic content PEF (256 kJ/kg): Total anthocyanins | No decreases | [42] | |
| Apple, grape, and orange juices | High pressure homogenization (HPH) (250 MPa for 10 min) | No information provided about structure | Apple juice: ↓ Total phenolic content, chlorogenic acid, phloridzin, epigallocatechin-3-gallate (EGCG) and hesperidin Grape juice: ↑ Total phenolic content, caffeoyl-trataric acid, proanthocyanidin; ↓ epicatechin protocatechuic-glucoside Orange juice: ↑ Total phenolic content, naringin, caffeoyl glucoside, hesperetin- rutinoside, naringenin-trisaccharide, luteolin-rutinoside; ↓ quercetin- trisaccharide | Apple juice: No increases Grape juice: Caffeoyl-tartaric acid Orange juice: Naringin, naringenin-trisaccharide, luteolin-rutinoside, quercetin-trisaccharide | Apple juice: Chlorogenic acid, phloridzin, hesperidin and total phenolic content Grape juice: No decreases Orange juice: No decreases | [59] | |
| Calçots | US (40 kHz; 250 W for 0, 10, 25, 45 min) | Firmness was not significantly affected | No changes in total phenolic content | No increases | Total phenolic content | [60] | |
| Tomato, lettuce, green pepper, red pepper, zucchini | US (40 kHz; 250 W for 20 min) | No information provided about structure | ↑ Total phenolic content in all products | Total phenolic content in green pepper and lettuce | Total phenolic content in tomato, red pepper and zucchini | [61] | |
| Juçara based smoothie | US (220 W for 7 min) | Microstructure similar to untreated smoothie but higher particle size (D4,3) | No changes in total phenolic content nor total anthocyanins | Total anthocyanins | No decreases | [62] | |
| Carrot | PEF (five pulses of 3.5 kV/cm) | ↓ Firmness Degradation of cell walls | ↓ Total phenolic content, coumaroylquinic acid, caffeic acid, caffeic acid arab/xiloside, caffeoylshikimic acid, 3-, 4-, 5-caffeoylquinic acid, dicaffeoylquinic acid, caffeic acid derivative, ferulic acid glucoside, ferulic acid coumaroyl glucoside, ferulic acid caffeoyl glucoside ↑ Coumaric acid, caffeoylferuloylquinic acid, caffeic acid arabinoside glucoside, ferulic acid, 3-feruloylquinic acid | Total phenolic content, caffeoylshikimic acid, caffeoylferuloylquinic acid, isoferulic acid, ferulic acid glucoside, ferulic acid caffeoyl glucoside, quercetin-3-O-galactoside, | 3-, 4-, 5-caffeoylquinic acid, caffeic acid arabinoside glucoside, caffeic acid Glu acetyl glucoside, ferulic acid, feruloylquinic acid derivative | [30] | |
| Fruit juice-based beverage mixed with water | PEF (35 kV/cm for 1800 μs) High pressure processing (HPP) (400 MPa for 5 min) | No information provided about structure | PEF: ↑ Caffeic acid, ferulic acid ↓ Total phenolic content chlorogenic acid, p-coumaric acid, p-hydroxybenzoic acid, hesperidin, quercetin, rutin HPP: ↑ Caffeic acid ↓ Total phenolic content, p-coumaric acid, p-hydroxybenzoic acid, quercetin, rutin | PEF: Caffeic acid, p-coumaric, hesperidin, quercetin, rutin HPP: Total phenolic content, caffeic acid, p-coumaric, hesperidin, quercetin, rutin | PEF: Total phenolic content, chlorogenic acid, ferulic acid, p-hydroxybenzoic acid HPP: Ferulic acid | [63] | |
| Fruit juice-based beverage mixed with milk | PEF (35 kV/cm for 1800 μs) HPP (400 MPa for 5 min) | No information provided about structure | PEF and HPP: ↑ Total phenolic content, caffeic acid, chlorogenic acid, p-coumaric acid, p-hydroxybenzoic acid, hesperidin, naringenin, quercetin ↓ ferulic acid, rutin | PEF and HPP: Total phenolic content, caffeic acid, chlorogenic acid, ferulic acid, p-coumaric acid, p-hydroxybenzoic acid, hesperidin, quercetin, rutin | No decreases | [63] | |
| Fruit juice-based beverage mixed with soymilk | PEF (35 kV/cm for 1800 μs) HPP (400 MPa for 5 min) | No information provided about structure | PEF and HPP: ↑ Total phenolic content, caffeic acid, chlorogenic acid, p-coumaric, p-hydroxybenzoic acid, hesperidin, naringenin, quercetin, rutin ↓ ferulic acid | PEF: Total phenolic content, quercetin, rutin HPP: Total phenolic content, p-hydroxybenzoic acid, hesperidin, naringenin, rutin | PEF and HPP: p-coumaric acid | [63] | |
| Fruit juice-based beverage mixed with soymilk | PEF (35 kV/cm for 1800 μs) HPP (400 MPa for 5 min) | No information provided about structure | PEF: ↑ Total isoflavones, daidzin, genistin, glycitin, daidzein, genistein, HPP: ↑ Total isoflavones, daidzin, genistin, glycitin, daidzein, genistein, glycitein | PEF: Total isoflavones, daidzin, genistin, daidzein, genistein, HPP: Total isoflavones, daidzin, genistin, daidzein, genistein, glycitein | No decreases | [64] | |
| Pomelo and kiwi juices | HPH (250 MPa for 10 min) | No information provided about structure | Pomelo juice: ↑ Total phenolic content ↑ Naringenin-rutinoside, isorhamnetin-rutinoside, naringenin-rutinoside-glucoside, proanthocyanidin, proanthocyanidin-glucoside. Kiwi juice: ↑ quinic acid, chlorogenic acid, caffeoyl glucoside, EGC. ↓ sinensetin | No increases | Pomelo juice: Naringenin-rutinoside, isorhamnetin-rutinoside, feruloyl-glucoside, total phenolic content Kiwi juice: Quinic acid | [65] | |
| Apple | PEF and storage for 0 h and 24 h | 0.01 kJ/kg | Unaltered toughness | 0 h: ↓ 5-caffeoylquinic acid 24 h: ↑ 5-caffeoylquinic acid, total phenolic content | No increases | 0 h: 5-caffeoylquinic acid Total phenolic content | [58] |
| 1.8 kJ/kg | ↓ Toughness | 0 h and 24 h: ↓ 5-caffeoylquinic acid, 4-caffeoylquinic acid, p-coumaroylquinic acid, phloretin xyloglucoside, total phenolic content 24 h: ↓ Epicatechin | 0 h and 24 h: Phloretin xyloglucoside 24 h: Total phenolic content, epicatechin 5-caffeoylquinic acid, phloretin glycoside | 24 h: Quercetin glycoside, quercetin xyloside, quercetin galactoside, quercetin arabinoside | |||
| 7.3 kJ/kg | ↓ Toughness | 0 h and 24 h: ↓ 5-caffeoylquinic acid, 4-caffeoylquinic acid, p-coumaroylquinic acid, phloretin glucoside, phloretin xyloglucoside, total phenolic content 0 h: ↓ Epicatechin | 24 h: Total phenolic content | 0 h: 4-caffeoylquinic acid | |||
| Açai juice | US (0.9, 1.8, 2.7 and 3.6 kJ/cm3) | No information provided about structure | ↑ Total anthocyanins (3.6 kJ/cm3) | Total anthocyanins | No decreases | [46] | |
| Orange juice | HPH (150 MPa reaching 68 °C for 15 s) | No information provided about structure | ↑ Vicenin-2 ↓ Total flavanones, total flavonoids, apigenin-d, hesperidin | No increases | No decreases | [49] | |
| Mandarin juices | HPH (150 MPa reaching 68 °C for 15 s) | Cell rupture and ↓ particle size | No changes | Apigenin | No decreases | [34] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Gámez, G.; Elez-Martínez, P.; Martín-Belloso, O.; Soliva-Fortuny, R. Recent Advances toward the Application of Non-Thermal Technologies in Food Processing: An Insight on the Bioaccessibility of Health-Related Constituents in Plant-Based Products. Foods 2021, 10, 1538. https://doi.org/10.3390/foods10071538
López-Gámez G, Elez-Martínez P, Martín-Belloso O, Soliva-Fortuny R. Recent Advances toward the Application of Non-Thermal Technologies in Food Processing: An Insight on the Bioaccessibility of Health-Related Constituents in Plant-Based Products. Foods. 2021; 10(7):1538. https://doi.org/10.3390/foods10071538
Chicago/Turabian StyleLópez-Gámez, Gloria, Pedro Elez-Martínez, Olga Martín-Belloso, and Robert Soliva-Fortuny. 2021. "Recent Advances toward the Application of Non-Thermal Technologies in Food Processing: An Insight on the Bioaccessibility of Health-Related Constituents in Plant-Based Products" Foods 10, no. 7: 1538. https://doi.org/10.3390/foods10071538
APA StyleLópez-Gámez, G., Elez-Martínez, P., Martín-Belloso, O., & Soliva-Fortuny, R. (2021). Recent Advances toward the Application of Non-Thermal Technologies in Food Processing: An Insight on the Bioaccessibility of Health-Related Constituents in Plant-Based Products. Foods, 10(7), 1538. https://doi.org/10.3390/foods10071538