Efficacy and Safety of Aronia, Red Ginseng, Shiitake Mushroom, and Nattokinase Mixture on Insulin Resistance in Prediabetic Adults: A Randomized, Double-Blinded, Placebo-Controlled Trial
Abstract
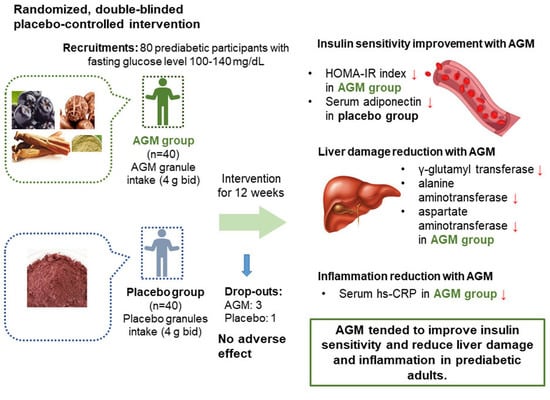
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Measurement of Indicative Compounds in AGM
2.3. Participants
2.4. Study Design
2.5. Sample Size Calculation
2.6. Intervention and Primary and Secondary Outcomes
2.7. Anthropometric Parameters, Blood Pressure Measurements, and Blood Collection
2.8. OGTT and Biochemical Assays
2.9. Dietary Intervention and Assessments of Dietary Intakes and Physical Activity Levels
2.10. Safety Assessment
2.11. Statistical Analysis
3. Results
3.1. Study Flow
3.2. Baseline Characteristics of the Participants
3.3. Nutrient Intake and BMI at Baseline and 12-Week Intervention and Compliance of Intervention
3.4. Efficacy Evaluation of Primary Outcomes
3.5. Evaluations of Liver Damage and Inflammation
3.6. Safety and Adverse Reactions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, S.; Nah, E.H.; Cho, S. Prevalence of Comorbidities among Patients with Diabetes. J. Health Inform. Stat. 2018, 43, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Ahn, I.S.; Kwon, D.Y.; Ko, B.S.; Jun, W.K. Ginsenosides Rb1 and Rg1 suppress triglyceride accumulation in 3T3-L1 adipocytes and enhance beta-cell insulin secretion and viability in Min6 cells via PKA-dependent pathways. Biosci. Biotechnol. Biochem. 2008, 72, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Shimizu, H.; Okazaki, Y.; Sakaguchi, H.; Taira, T.; Suzuki, T.; Chiji, H. Anthocyanin-rich Phytochemicals from Aronia Fruits Inhibit Visceral Fat Accumulation and Hyperglycemia in High-Fat Diet-Induced Dietary Obese Rats. J. Oleo Sci. 2015, 64, 1243–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, J.; Xin, G.; Zhang, B.; Wang, Y.; Ning, C.; Meng, X. Beneficial effects of Aronia melanocarpa berry extract on hepatic insulin resistance in type 2 diabetes mellitus rats. J. Food Sci. 2020, 85, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Jurgoński, A.; Juśkiewicz, J.; Zduńczyk, Z. Ingestion of black chokeberry fruit extract leads to intestinal and systemic changes in a rat model of prediabetes and hyperlipidemia. Plant. Foods Hum. Nutr. 2008, 63, 176–182. [Google Scholar] [CrossRef]
- Vuksan, V.; Xu, Z.Z.; Jovanovski, E.; Jenkins, A.L.; Beljan-Zdravkovic, U.; Sievenpiper, J.L.; Mark Stavro, P.; Zurbau, A.; Duvnjak, L.; Li, M.Z.C. Efficacy and safety of American ginseng (Panax quinquefolius L.) extract on glycemic control and cardiovascular risk factors in individuals with type 2 diabetes: A double-blind, randomized, cross-over clinical trial. Eur. J. Nutr. 2019, 58, 1237–1245. [Google Scholar] [CrossRef]
- Kanwal, S.; Aliya, S.; Xin, Y. Anti-Obesity Effect of Dictyophora indusiata Mushroom Polysaccharide (DIP) in High Fat Diet-Induced Obesity via Regulating Inflammatory Cascades and Intestinal Microbiome. Front. Endocrinol. 2020, 11, 558874. [Google Scholar] [CrossRef] [PubMed]
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Awasthi, A. Therapeutic potential of mushrooms in diabetes mellitus: Role of polysaccharides. Int. J. Biol. Macromol. 2020, 164, 1194–1205. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.S.; Kang, S. Vitamin D deficiency impairs glucose-stimulated insulin secretion and increases insulin resistance by reducing PPAR-γ expression in nonobese Type 2 diabetic rats. J. Nutr. Biochem. 2016, 27, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ham, J.O.; Lee, B.K. A positive association of vitamin D deficiency and sarcopenia in 50-year-old women, but not men. Clin. Nutr. 2014, 33, 900–905. [Google Scholar] [CrossRef]
- Borel, P.; Caillaud, D.; Cano, N.J. Vitamin D bioavailability: State of the art. Crit. Rev. Food Sci. Nutr. 2015, 55, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.S.; Lenninger, M.; Ero, M.P.; Benson, K.F. Consumption of nattokinase is associated with reduced blood pressure and von Willebrand factor, a cardiovascular risk marker: Results from a randomized, double-blind, placebo-controlled, multicenter North American clinical trial. Integr. Blood Press Control 2016, 9, 95–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.J.; Kim, M.J.; Kwon, D.Y.; Kim, D.S.; Zhang, T.; Ha, C.; Park, S. Combination of Aronia, Red Ginseng, Shiitake Mushroom and Nattokinase Potentiated Insulin Secretion and Reduced Insulin Resistance with Improving Gut Microbiome Dysbiosis in Insulin Deficient Type 2 Diabetic Rats. Nutrients 2018, 10, 948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Kang, S.; Jeong, D.-Y.; Jeong, S.-Y.; Park, J.J.; Yun, H.S. Cyanidin and malvidin in aqueous extracts of black carrots fermented with Aspergillus oryzae prevent the impairment of energy, lipid, and glucose metabolism in estrogen-deficient rats by AMPK activation. Genes Nutr. 2015, 10, 6. [Google Scholar] [CrossRef]
- Brown, P.N.; Yu, R. Collaborators: Determination of Ginsenoside Content in Panax ginseng C.A. Meyer and Panax quinquefolius L. Root Materials and Finished Products by High-Performance Liquid Chromatography with Ultraviolet Absorbance Detection: Interlaboratory Study. J. AOAC Int. 2019, 96, 12–19. [Google Scholar] [CrossRef]
- Mehrotra, A.; Calvo, M.S.; Beelman, R.B.; Levy, E.; Siuty, J.; Kalaras, M.D.; Uribarri, J. Bioavailability of vitamin D 2 from enriched mushrooms in prediabetic adults: A randomized controlled trial. Eur. J. Clin. Nutr. 2014, 68, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, E.; Solis-Herrera, C.; Trautmann, M.E.; Malloy, J.; Triplitt, C.L. Assessment of pancreatic β-cell function: Review of methods and clinical applications. Curr. Diab. Rev. 2014, 10, 2–42. [Google Scholar] [CrossRef] [Green Version]
- Furugen, M.; Saitoh, S.; Ohnishi, H.; Akasaka, H.; Mitsumata, K.; Chiba, M.; Furukawa, T.; Miyazaki, Y.; Shimamoto, K.; Miura, T. Matsuda–DeFronzo insulin sensitivity index is a better predictor than HOMA-IR of hypertension in Japanese: The Tanno–Sobetsu study. J. Hum. Hypertens 2012, 26, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Ram, J.; Snehalatha, C.; Selvam, S.; Nanditha, A.; Shetty, A.S.; Godsland, I.F.; Johnston, D.G.; Ramachandran, A. The oral disposition index is a strong predictor of incident diabetes in Asian Indian prediabetic men. Acta Diabetol. 2015, 52, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Edirisinghe, I.; Burton-Freeman, B. Anti-diabetic actions of Berry polyphenols—Review on proposed mechanisms of action. J. Berry Res. 2016, 6, 237–250. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Balan, P.; Popovich, D.G. Review of Ginseng Anti-Diabetic Studies. Molecules 2019, 24, 4501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Kang, S.; Kim, D.S. Severe calcium deficiency increased visceral fat accumulation, down-regulating genes associated with fat oxidation, and increased insulin resistance while elevating serum parathyroid hormone in estrogen-deficient rats. Nutr. Res. 2020, 73, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Wondmkun, Y.T. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab. Syndr. Obes. 2020, 13, 3611–3616. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, M.H.; Kim, S.H. Early gestational weight gains within current recommendations result in increased risk of gestational diabetes mellitus among Korean women. Diabetes Metab. Res. Rev. 2014, 30, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, G.; Ding, M.; Zong, G.; Hu, F.B.; Willett, W.C.; Rimm, E.B.; Manson, J.E.; Sun, Q. Isoflavone Intake and the Risk of Coronary Heart Disease in US Men and Women. Circulation 2020, 141, 1127–1137. [Google Scholar] [CrossRef]
- Yamane, T.; Kozuka, M.; Konda, D.; Nakano, Y.; Nakagaki, T.; Ohkubo, I.; Ariga, H. Improvement of blood glucose levels and obesity in mice given Aronia juice by inhibition of dipeptidyl peptidase IV and α-glucosidase. J. Nutr. Biochem. 2016, 31, 106–112. [Google Scholar] [CrossRef]
- Karmazyn, M.; Gan, X.T. Ginseng for the treatment of diabetes and diabetes-related cardiovascular complications: A discussion of the evidence (1). Can. J. Physiol. Pharm. 2019, 97, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Barchetta, I.; Cimini, F.A.; Cavallo, M.G. Vitamin D Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD): An Update. Nutrients 2020, 12, 3302. [Google Scholar] [CrossRef]
- Chiang, J.M.; Stanczyk, F.Z.; Kanaya, A.M. Vitamin D Levels, Body Composition, and Metabolic Factors in Asian Indians: Results from the Metabolic Syndrome and Atherosclerosis in South Asians Living in America Pilot Study. Ann. Nutr. Metab. 2018, 72, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Wang, X.; Wang, N.; Li, Q.; Chen, Y.; Zhu, C.; Chen, Y.; Xia, F.; Pu, X.; Cang, Z.; et al. Investigation of vitamin D status and its correlation with insulin resistance in a Chinese population. Public Health Nutr. 2017, 20, 1602–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pramono, A.; Jocken, J.W.E.; Blaak, E.E.; Van Baak, M.A. The Effect of Vitamin D Supplementation on Insulin Sensitivity: A Systematic Review and Meta-analysis. Diabetes Care 2020, 43, 1659–1669. [Google Scholar] [CrossRef] [PubMed]
- Mirhosseini, N.; Vatanparast, H.; Mazidi, M.; Kimball, S.M. The Effect of Improved Serum 25-Hydroxyvitamin D Status on Glycemic Control in Diabetic Patients: A Meta-Analysis. J. Clin. Endocrinol. Metab. 2017, 102, 3097–3110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, D.; Chen, W.; Li, X.; Yue, T.; Zhang, Z.; Feng, Z.; Li, C.; Bu, X.; Li, Q.X.; Hu, C.Y.; et al. Ultraviolet Irradiation Increased the Concentration of Vitamin D2 and Decreased the Concentration of Ergosterol in Shiitake Mushroom (Lentinus edodes) and Oyster Mushroom (Pleurotus ostreatus) Powder in Ethanol Suspension. ACS Omega 2020, 5, 7361–7368. [Google Scholar] [CrossRef] [PubMed]
- Thacher, T.D.; Fischer, P.R.; Obadofin, M.O.; Levine, M.A.; Singh, R.J.; Pettifor, J.M. Comparison of metabolism of vitamins D2 and D3 in children with nutritional rickets. J. Bone Miner. Res. 2010, 25, 1988–1995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, D.M.; Leder, B.Z.; Cagliero, E.; Mendoza, N.; Henao, M.P.; Hayden, D.L.; Finkelstein, J.S.; Burnett-Bowie, S.-A.M. Insulin secretion and sensitivity in healthy adults with low vitamin D are not affected by high-dose ergocalciferol administration: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 385–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, D.Y.; Jeong, S.Y.; Zhang, T.; Wu, X.; Qiu, J.Y.; Park, S. Chungkookjang, a soy food, fermented with Bacillus amyloliquefaciens protects gerbils against ishcmeic stroke injury, and post-stroke hyperglycemia. Food Res. Int. 2020, 128, 108769. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, K.J.; Kim, S. Purification and Antithrombotic Potential of a Fibrinolytic Enzyme from Shiitake Culinary-Medicinal Mushroom, Lentinus edodes GNA01 (Agaricomycetes). Int. J. Med. Mushrooms 2018, 20, 47–59. [Google Scholar] [CrossRef]
- Ooi, S.L.; Pak, S.C.; Micalos, P.S.; Schupfer, E.; Lockley, C.; Park, M.H.; Hwang, S.J. The Health-Promoting Properties and Clinical Applications of Rice Bran Arabinoxylan Modified with Shiitake Mushroom Enzyme-A Narrative Review. Molecules 2021, 26, 2539. [Google Scholar] [CrossRef]
- Gresele, P.; Marzotti, S.; Guglielmini, G.; Momi, S.; Giannini, S.; Minuz, P.; Lucidi, P.; Bolli, G.B. Hyperglycemia-induced platelet activation in type 2 diabetes is resistant to aspirin but not to a nitric oxide-donating agent. Diabetes Care 2010, 33, 1262–1268. [Google Scholar] [CrossRef] [Green Version]
- Drori, A.; Rotnemer-Golinkin, D.; Avni, S.; Drori, A.; Danay, O.; Levanon, D.; Tam, J.; Zolotarev, L.; Ilan, Y. Attenuating the rate of total body fat accumulation and alleviating liver damage by oral administration of vitamin D-enriched edible mushrooms in a diet-induced obesity murine model is mediated by an anti-inflammatory paradigm shift. BMC Gastroenterol. 2017, 17, 130. [Google Scholar] [CrossRef] [Green Version]
- Jayachandran, M.; Chen, J.; Chung, S.S.M.; Xu, B. A critical review on the impacts of β-glucans on gut microbiota and human health. J. Nutr. Biochem. 2018, 61, 101–110. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.-Y.; Wei, Y.-L.; Hao, J.-Y.; Lei, Y.-Q.; Zhao, W.-B.; Xiao, Y.-H.; Sun, A.-D. The polyphenol-rich extract from chokeberry (Aronia melanocarpa L.) modulates gut microbiota and improves lipid metabolism in diet-induced obese rats. Nutr. Metab. 2020, 17, 54. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-J.; Zhang, T.; Wu, X.-G.; Kim, M.-J.; Kim, Y.-H.; Yang, E.-S.; Yoon, Y.-S.; Park, S. Aqueous Blackcurrant Extract Improves Insulin Sensitivity and Secretion and Modulates the Gut Microbiome in Non-Obese Type 2 Diabetic Rats. Antioxidants 2021, 10, 756. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, F.; Ducluzeau, P.H.; Gastaldelli, A.; Laville, M.; Anderwald, C.H.; Konrad, T.; Mari, A.; Balkau, B. Liver enzymes are associated with hepatic insulin resistance, insulin secretion, and glucagon concentration in healthy men and women. Diabetes 2011, 60, 1660–1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehrke, N.; Schattenberg, J.M. Metabolic Inflammation-A Role for Hepatic Inflammatory Pathways as Drivers of Comorbidities in Nonalcoholic Fatty Liver Disease? Gastroenterology 2020, 158, 1929–1947.e6. [Google Scholar] [CrossRef] [PubMed]
- Pyun, C.W.; Seo, T.S.; Kim, D.J.; Kim, T.W.; Bae, J.S. Protective Effects of Ligularia fischeri and Aronia melanocarpa Extracts on Alcoholic Liver Disease (In Vitro and In Vivo Study). Biomed. Res. Int. 2020, 2020, 9720387. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Kim, K.; Lee, B.K.; Ahn, J. A Healthy Diet Rich in Calcium and Vitamin C Is Inversely Associated with Metabolic Syndrome Risk in Korean Adults from the KNHANES 2013–2017. Nutrients 2021, 13, 1312. [Google Scholar] [CrossRef]
- Zhou, J.-Y.; Park, S. Associations of Polygenetic Variants at the 11q23 Locus and Their Interactions with Macronutrient Intake for the risk of 3GO, a Combination of Hypertension, Hyperglycemia, and Dyslipidemia. J. Pers. Med. 2021, 11, 207. [Google Scholar] [CrossRef]
- Jung, C.H.; Choi, K.M. Impact of High-Carbohydrate Diet on Metabolic Parameters in Patients with Type 2 Diabetes. Nutrients 2017, 9, 322. [Google Scholar] [CrossRef]
- Dinu, M.; Pagliai, G.; Angelino, D.; Rosi, A.; Dall’Asta, M.; Bresciani, L.; Ferraris, C.; Guglielmetti, M.; Godos, J.; Del Bo, C.; et al. Effects of Popular Diets on Anthropometric and Cardiometabolic Parameters: An Umbrella Review of Meta-Analyses of Randomized Controlled Trials. Adv. Nutr. 2020, 11, 815–833. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kang, S. High carbohydrate and noodle/meat-rich dietary patterns interact with the minor haplotype in the 22q13 loci to increase its association with non-alcoholic fatty liver disease risk in Koreans. Nutr. Res. 2020, 82, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Shin, S.; Ha, K.; Hwang, Y.; Park, Y.H.; Kang, M.S.; Joung, H. Effect of a balanced Korean diet on metabolic risk factors among overweight/obese Korean adults: A randomized controlled trial. Eur. J. Nutr. 2020, 59, 3023–3035. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Unno, T.; Kang, S.; Park, S. A Korean-Style Balanced Diet Has a Potential Connection with Ruminococcaceae Enterotype and Reduction of Metabolic Syndrome Incidence in Korean Adults. Nutrients 2021, 13, 495. [Google Scholar] [CrossRef] [PubMed]
- Schisterman, E.F.; Mumford, S.L.; Sjaarda, L.A. Failure to consider the menstrual cycle phase may cause misinterpretation of clinical and research findings of cardiometabolic biomarkers in premenopausal women. Epidemiol. Rev. 2014, 36, 71–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| AGM (n = 40) | Placebo (n = 40) | p Value (1) | |
|---|---|---|---|
| Genders (M/F) | 24/16 | 31/9 | 0.091 (2) |
| Age (years) | 42.0 ± 12.7 | 39.2 ± 12.3 | 0.308 |
| Height (cm) | 166.5 ± 9.7 | 170.0 ± 7.7 | 0.076 |
| Weight (kg) | 67.6 ± 12.5 | 73.7 ± 13.3 | 0.035 * |
| BMI (kg/m2) | 24.2 ± 2.71 | 25.4 ± 3.32 | 0.099 |
| Serum glucose at fasting (mg/dL) | 105.5 ± 9.20 | 108.6 ± 10.7 | 0.187 |
| Serum insulin at fasting (μU/mL) | 8.36 ± 7.83 | 8.13 ± 4.74 | 0.88 |
| SBP (mmHg) | 126.9 ± 13.2 | 128.2 ± 10.1 | 0.622 |
| DBP (mmHg) | 80.2 ± 11.2 | 80.58 ± 9.75 | 0.873 |
| Pulse (per minute) | 75.6 ± 12.0 | 78.03 ± 8.64 | 0.304 |
| Alcohol (n, %) | 18 (45) | 18 (45) | 1.000 (2) |
| Alcohol (units/week) | 9.58 ± 3.92 | 9.14 ± 5.04 | 0.772 |
| Smoking (n, %) | 4 (10) | 10 (25) | 0.078 (2) |
| Smoking (cigarette/day) | 15.0 ± 5.77 | 11.9 ± 5.0 | 0.334 |
| AGM | Placebo | ||||
|---|---|---|---|---|---|
| Baseline (n = 37) | 12-Week (n = 37) | Placebo (n = 39) | 12-Week (n = 39) | p Value (2) | |
| Energy intake (kcal) | 1722 ± 457 (1) | 1665 ± 478 | 1788 ± 554 | 1702 ± 547 | 0.768 |
| Carbohydrate intake (En %) | 56.9 ± 17.2 | 58.0 ± 17.2 | 56.3 ± 15.2 | 54.0 ± 15.2 | 0.607 |
| Protein intake (En %) | 17.0 ± 4.9 | 16.7 ± 6.2 | 17.5 ± 6.0 | 17.5 ± 6.1 | 0.902 |
| Fat intake (En %) | 26.1 ± 10.6 | 25.3 ± 9.8 | 29.7 ± 13.0 | 28.5 ± 13.0 | 0.917 |
| Fiber intake (En %) | 19.7 ± 7.8 | 18.6 ± 7.9 | 18.6 ± 7.5 | 17.7 ± 6.5 | 0.890 |
| Vitamin A (ug RE) | 794 ± 389 | 747 ± 370 | 759 ± 443 | 767 ± 354 | 0.553 |
| Vitamin E (mg) | 14.6 ± 6.80 | 15.7 ± 8.25 | 16.6 ± 8.58 | 15.9 ± 6.66 | 0.290 |
| Vitamin C (mg) | 99.6 ± 59.0 | 88.9 ± 49.4 | 86.5 ± 48.7 | 89.8 ± 42.6 | 0.137 |
| Zinc (mg) | 10.0 ± 2.96 | 9.51 ± 3.01 | 10.5 ± 3.47 | 10.1 ± 3.96 | 0.829 |
| Selenium (ug) | 94.1 ± 28.8 | 87.3 ± 30.6 | 97.8 ± 33.3 | 92.9 ± 34.0 | 0.791 |
| Alcohol (units/week) | 9.70 ± 4.02 | 6.81 ± 3.00 | 9.14 ± 5.04 | 7.03 ± 3.49 | 0.268 |
| Smoking (cigarette/day) | 16.7 ± 5.8 | 13.3 ± 7.6 | 11.9 ± 5.0 | 11.0 ± 4.4 | 0.563 |
| Physical activity (min/week) | 5572 ± 12725 | 4664 ± 7476 | 2874 ± 4465 | 4196 ± 5835 | 0.082 |
| Body weight (kg) | 67.8 ± 12.6 | 67.8 ± 12.6 | 74.0 ± 13.3 | 74.0 ± 13.6 | 0.945 |
| Body mass index (kg/m2) | 24.2 ± 2.75 | 24.2 ± 2.67 | 25.5 ± 3.31 | 25.5 ± 3.42 | 0.780 |
| Compliance of taking supplements (%) (3) | - | 94.8 ± 4.33 | - | 96.0 ± 4.71 | 0.260 |
| Time | ARC Group (n = 37) | Placebo Group (n = 39) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12-Week | Change Value | p Value (2) | Baseline | 12-Week | Change Value | p Value (2) | p Value (3) | Adjusted p Value (4) | |
| Serum glucose (mg/dL) | ||||||||||
| 0 min | 105.5 ± 9.2 (1) | 105.1 ± 10.2 | −0.37 ± 9.7 | 0.817 | 108.6 ± 10.7 | 108.7 ± 15.0 | 0.09 ± 12.8 | 0.964 | 0.859 | 0.859 |
| 30 min | 173.5 ± 32.2 | 175.1 ± 29.4 | 1.57 ± 27.4 | 0.729 | 182.1 ± 23.7 | 182.1 ± 30.3 | 0.03 ± 26.1 | 0.995 | 0.802 | 0.802 |
| 60 min | 172.4 ± 46.2 | 168.3 ± 43.9 | −4.09 ± 29.4 | 0.402 | 187.1 ± 45.4 | 194.6 ± 45.4 | 7.48 ± 42.2 | 0.276 | 0.168 | 0.172 |
| 90 min | 153.0 ± 48.1 | 159.2 ± 48.3 | 6.17 ± 30.7 | 0.229 | 168.0 ± 53.0 | 173.0 ± 52.2 | 4.97 ± 38.3 | 0.422 | 0.881 | 0.881 |
| 120 min | 137.7 ± 49.3 | 138.0 ± 49.7 | 0.33 ± 30.8 | 0.949 | 150.7 ± 46.0 | 158.6 ± 49.3 | 7.89 ± 44.2 | 0.272 | 0.388 | 0.392 |
| p value | <0.001 (5) | <0.001 (5) | 0.043 (6) | 0.043 (7) | ||||||
| Serum insulin (uU/mL) | ||||||||||
| 0 min | 8.36 ± 7.83 | 5.29 ± 2.17 | −3.07 ± 7.06 | 0.012 | 8.13 ± 4.74 | 8.18 ± 8.04 | 0.05 ± 6.12 | 0.962 | 0.043 | 0.043 |
| 30 min | 44.8 ± 36.1 | 44.4 ± 27.9 | −0.39 ± 24.9 | 0.925 | 50.0 ± 41.5 | 50.8 ± 33.9 | 0.73 ± 24.8 | 0.856 | 0.846 | 0.846 |
| 60 min | 43.0 ± 20.0 | 40.9 ± 22.3 | −2.11 ± 24.5 | 0.603 | 56.3 ± 35.7 | 59.5 ± 37.1 | 3.18 ± 32.9 | 0.55 | 0.431 | 0.431 |
| 90 min | 38.3 ± 20.2 | 46.1 ± 31.6 | 7.79 ± 29.6 | 0.118 | 51.0 ± 30.2 | 51.4 ± 32.5 | 0.34 ± 30.9 | 0.946 | 0.287 | 0.287 |
| 120 min | 37.6 ± 30.8 | 34.4 ± 22.8 | −3.19 ± 33.2 | 0.563 | 42.1 ± 26.0 | 46.3 ± 27.7 | 4.15 ± 31.2 | 0.41 | 0.323 | 0.323 |
| p value | <0.001 (5) | <0.001 (5) | 0.162 (6) | 0.162 (7) | ||||||
| Area under curve | ||||||||||
| Glucose (mg*min/dL) | 6011 ± 3531 | 6137 ± 3284 | 126.6 ± 2188 | 0.727 | 6991 ± 3114 | 7471 ± 3428 | 480.0 ± 2714 | 0.276 | 0.535 | 0.535 |
| Insulin | 3477 ± 1819 | 3904 ± 1842 | 426.3 ± 1733 | 0.143 | 4499 ± 2755 | 4708 ± 2911 | 209.2 ± 2308 | 0.575 | 0.646 | 0.646 |
| (μU*min/mL) | ||||||||||
| Time | ARC Group (n = 37) | Placebo Group (n = 39) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12-Week | Change Value | p Value (2) | Baseline | 12-Week | Change Value | p Value (2) | p Value (3) | Adjusted p Value (4) | |
| HbA1c (%) | 5.52 ± 0.38 (1) | 5.57 ± 0.38 | 0.05 ± 0.20 | 0.141 | 5.63 ± 0.30 | 5.63 ± 0.42 | 0.00 ± 0.29 | 0.971 | 0.373 | 0.377 |
| C-peptide (ng/mL) | 2.40 ± 1.10 | 1.90 ± 0.53 | −0.49 ± 1.00 | 0.005 | 2.49 ± 1.03 | 2.30 ± 1.10 | −0.19 ± 0.71 | 0.108 | 0.132 | 0.128 |
| HOMA-IR | 2.24 ± 2.31 | 1.39 ± 0.60 | −0.85 ± 2.14 | 0.020 | 2.19 ± 1.35 | 2.26 ± 2.56 | 0.07 ± 1.92 | 0.812 | 0.049 | 0.049 |
| Matsuda index | 6.32 ± 3.65 | 6.90 ± 4.04 | 0.58 ± 3.28 | 0.289 | 5.12 ± 2.63 | 5.32 ± 3.07 | 0.20 ± 2.77 | 0.651 | 0.588 | 0.588 |
| HOMA-B | 69.2 ± 54.8 | 46.4 ± 19.5 | −22.8 ± 46.2 | 0.005 | 66.9 ± 41.0 | 64.2 ± 46.1 | −2.77 ± 39.0 | 0.661 | 0.044 | 0.044 |
| Oral disposition index | 2.76 ± 1.73 | 3.60 ± 2.59 | 0.84 ± 2.27 | 0.031 | 2.26 ± 1.25 | 2.70 ± 2.82 | 0.43 ± 2.24 | 0.234 | 0.433 | 0.433 |
| Serum adiponectin (ng/mL) | 55.2 ± 37.1 | 55.3 ± 38.1 | 0.13 ± 12.7 | 0.95 | 49.4 ± 44.5 | 45.9 ± 39.2 | −3.42 ± 10.6 | 0.052 | 0.19 | 0.19 |
| AGM Group (n = 37) | Placebo Group (n = 39) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12-Week | Change Value | p Value (2) | Baseline | 12-Week | Change Value | p Value (2) | p Value (3) | |
| γ-GT (U/L) | 28.8 ± 24.7 (1) | 23.9 ± 16.4 | −4.90 ± 12.4 | 0.022 * | 33.6 ± 24.7 | 34.4 ± 25.7 | 0.83 ± 14.4 | 0.721 | 0.068 |
| AST (U/L) | 26.6 ± 9.0 | 21.8 ± 5.2 | −4.77 ± 8.96 | 0.003 ** | 24.9 ± 6.6 | 24.3 ± 8.0 | −0.58 ± 6.12 | 0.556 | 0.021 * |
| ALT (U/L) | 27.1 ± 13.8 | 20.6 ± 10.0 | −6.48 ± 10.7 | 0.001 ** | 28.9 ± 15.6 | 29.4 ± 18.1 | 0.52 ± 14.6 | 0.824 | 0.020 * |
| hs-CRP (mg/L) | 0.87 ± 0.78 | 0.72 ± 0.64 | −0.15 ± 0.84 | 0.299 | 0.85 ± 0.99 | 1.34 ± 2.02 | 0.50 ± 1.98 | 0.126 | 0.069 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Kim, C.-J.; Ha, K.-C.; Baek, H.-I.; Yang, H.-J.; Kim, M.-J.; Park, S.-J. Efficacy and Safety of Aronia, Red Ginseng, Shiitake Mushroom, and Nattokinase Mixture on Insulin Resistance in Prediabetic Adults: A Randomized, Double-Blinded, Placebo-Controlled Trial. Foods 2021, 10, 1558. https://doi.org/10.3390/foods10071558
Park S, Kim C-J, Ha K-C, Baek H-I, Yang H-J, Kim M-J, Park S-J. Efficacy and Safety of Aronia, Red Ginseng, Shiitake Mushroom, and Nattokinase Mixture on Insulin Resistance in Prediabetic Adults: A Randomized, Double-Blinded, Placebo-Controlled Trial. Foods. 2021; 10(7):1558. https://doi.org/10.3390/foods10071558
Chicago/Turabian StylePark, Sunmin, Chan-Joong Kim, Ki-Chan Ha, Hyang-Im Baek, Hye-Jeong Yang, Min-Jung Kim, and Soo-Jung Park. 2021. "Efficacy and Safety of Aronia, Red Ginseng, Shiitake Mushroom, and Nattokinase Mixture on Insulin Resistance in Prediabetic Adults: A Randomized, Double-Blinded, Placebo-Controlled Trial" Foods 10, no. 7: 1558. https://doi.org/10.3390/foods10071558
APA StylePark, S., Kim, C. -J., Ha, K. -C., Baek, H. -I., Yang, H. -J., Kim, M. -J., & Park, S. -J. (2021). Efficacy and Safety of Aronia, Red Ginseng, Shiitake Mushroom, and Nattokinase Mixture on Insulin Resistance in Prediabetic Adults: A Randomized, Double-Blinded, Placebo-Controlled Trial. Foods, 10(7), 1558. https://doi.org/10.3390/foods10071558