Encapsulation and Protection of Omega-3-Rich Fish Oils Using Food-Grade Delivery Systems
Abstract
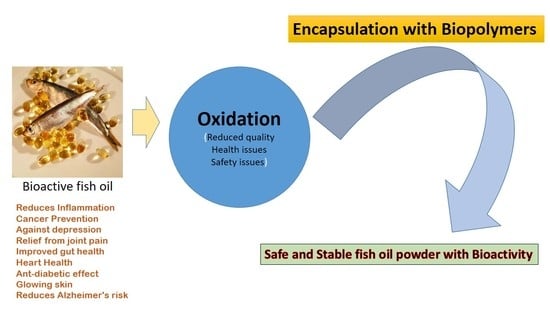
:1. Introduction
2. Marine Lipids—Physiological Significance and Potential Health Benefits
2.1. Inflammation
2.2. Oxidation
2.3. Lipid Profile
2.4. Cardiovascular Diseases
2.5. Thrombosis
2.6. Diabetes
2.7. Rheumatoid Arthritis
2.8. Ulcerative Colitis
3. Recommended Intake of DHA and EPA
4. Extraction and Characterization of Fish Oil Lipids
4.1. Extraction Methods
4.1.1. Traditional Methods
4.1.2. Green Methods
4.2. Characterization Methods
5. Challenges to Fish Oil Incorporation into Foods
6. Encapsulation
6.1. Encapsulation Technologies
6.1.1. Liposomes
6.1.2. Emulsions and Nanoemulsions
6.1.3. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers
6.1.4. Multiple Emulsions
6.1.5. Microgels
6.1.6. Nanofibers
6.1.7. Inclusion Complexes
7. Microencapsulation
7.1. Wall Materials
- Carbohydrates: maltodextrin, sucrose, corn syrup solids, modified starch, gum arabic, agar, alginates, carrageenan, pectin, and chitosan.
- Proteins: skimmed milk powder, gelatin, sodium caseinate, and whey protein.
| Wall Materials | Percentage of Wall Materials | Encapsulation Efficiency | Reference |
|---|---|---|---|
| WPI + CS+ MD for tuna oil | CS (0.5, 1, 1.5% w/w) MD (1% w/w), WPI (10% w/w) | 80–86% | [90] |
| WPI for fish oil | WPI (1:2), SPI (3:1) | WPI—97% SPI—93% | [91] |
| CS + lecithin for tuna oil | CS (0.2% w/w) Lecithin (1% w/w) | 87% | [92] |
| WPI + MD | 90:10, 50:50, 10:90 | 45–65% | [93] |
| GA WPI GA + WPI for cardamom oil | 100 g 100 g GA + WPI(1:1) GA + WPI ( 3:1) | 92% 69.2% 83.3% 74.3% | [94] |
7.2. Microencapsulation Technologies
7.2.1. Spray Drying
7.2.2. Freeze Drying
7.2.3. Extrusion
7.2.4. Electro-Spraying and Electro-Spinning
8. Characterization of Encapsulated Microparticles
9. Digestibility of Encapsulated Fish Oil
10. Conclusions
- Fish oil is rich in health-promoting omega-3 polyunsaturated fatty acids (PUFAs)
- PUFAs are difficult to incorporate into foods due to low water-solubility and chemical stability
- Encapsulation technologies can be used to overcome dispersibility and stability issues
- Novel and conventional encapsulation technologies are reviewed.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heileson, J.L.; Funderburk, L.K. The effect of fish oil supplementation on the promotion and preservation of lean body mass, strength, and recovery from physiological stress in young, healthy adults: A systematic review. Nutr. Rev. 2020, 78, 1001–1014. [Google Scholar] [CrossRef]
- Mesa, M.D.; Gil, F.; Olmedo, P.; Gil, A. Nutritional Importance of Selected Fresh Fishes, Shrimps and Mollusks to Meet Compliance with Nutritional Guidelines of n-3 LC-PUFA Intake in Spain. Nutrients 2021, 13, 465. [Google Scholar] [CrossRef]
- Salman, I.S. The effect of fish oil and omega-3 fatty acid on some physiological and Biochemical Criteria in Male Rabbits. Al-Nahrain J. Sci. 2017, 20, 108–113. [Google Scholar] [CrossRef]
- Zhao, W.; Tang, H.; Yang, X.; Luo, X.; Wang, X.; Shao, C.; He, J. Fish consumption and stroke risk: A meta-analysis of prospective cohort studies. J. Stroke Cerebrovasc. Dis. 2019, 28, 604–611. [Google Scholar] [CrossRef]
- Jedrusek-Golinska, A.; Górecka, D.; Buchowski, M.; Wieczorowska-Tobis, K.; Gramza-Michałowska, A.; Szymandera-Buszka, K. Recent progress in the use of functional foods for older adults: A narrative review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 835–856. [Google Scholar] [CrossRef]
- Khoshnoudi-Nia, S.; Forghani, Z.; Jafari, S.M. A systematic review and meta-analysis of fish oil encapsulation within different micro/nanocarriers. Crit. Rev. Food Sci. Nutr. 2020, 1–22. [Google Scholar] [CrossRef]
- Grootveld, M.; Percival, B.C.; Leenders, J.; Wilson, P.B. Potential adverse public health effects afforded by the ingestion of dietary lipid oxidation product toxins: Significance of fried food sources. Nutrients 2020, 12, 974. [Google Scholar] [CrossRef] [Green Version]
- Bannenberg, G.; Mallon, C.; Edwards, H.; Yeadon, D.; Yan, K.; Johnson, H.; Ismail, A. Omega-3 long-chain polyunsaturated fatty acid content and oxidation state of fish oil supplements in New Zealand. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmmed, M.K.; Ahmmed, F.; Tian, H.; Carne, A.; Bekhit, A.E.D. Marine omega-3 (n-3) phospholipids: A comprehensive review of their properties, sources, bioavailability, and relation to brain health. Compr. Rev. Food Sci. Food Saf. 2020, 19, 64–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClements, D.J.; Öztürk, B. Utilization of Nanotechnology to Improve the Handling, Storage and Biocompatibility of Bioactive Lipids in Food Applications. Foods 2021, 10, 365. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Ying, D.; Hlaing, M.M.; Ye, J.; Sanguansri, L.; Augustin, M.A. Oxidative stability of spray dried matcha-tuna oil powders. Food Res. Int. 2020, 132, 109050. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.M. Advances in spray-drying encapsulation of food bioactive ingredients: From microcapsules to nanocapsules. Annu. Rev. Food Sci. Technol. 2019, 10, 103–131. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Rundblad, A.; Holven, K.B.; Bruheim, I.; Myhrstad, M.C.; Ulven, S.M. Effects of fish and krill oil on gene expression in peripheral blood mononuclear cells and circulating markers of inflammation: A randomised controlled trial. J. Nutr. Sci. 2018, 7. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, T.B.; Rudd, D.; Kotiw, M.; Liu, L.; Benkendorff, K. Correlation between fatty acid profile and anti-inflammatory activity in common Australian seafood by-products. Mar. Drugs 2019, 17, 155. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Meng, Y.; Li, N.; Wang, Q.; Chen, L. The effects of low-ratio n-6/n-3 PUFA on biomarkers of inflammation: A systematic review and meta-analysis. Food Funct. 2020, 12, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, F.; Ramnath, N.; Nagrath, D. Reactive oxygen species in the tumor microenvironment: An overview. Cancers 2019, 11, 1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanna, K.; Kohli, S.K.; Bali, S.; Kaur, P.; Saini, P.; Bakshi, P.; Bhardwaj, R. Role of micro-organisms in modulating antioxidant defence in plants exposed to metal toxicity. In Plants Under Metal and Metalloid Stress; Springer: Singapore, 2018; pp. 303–335. [Google Scholar]
- Van Houten, B.; Santa-Gonzalez, G.A.; Camargo, M. DNA repair after oxidative stress: Current challenges. Curr. Opin. Toxicol. 2018, 7, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Adwas, A.A.; Elsayed, A.; Azab, A.E.; Quwaydir, F.A. Oxidative stress and antioxidant mechanisms in human body. J. Appl. Biotechnol. Bioeng. 2019, 6, 43. [Google Scholar]
- Tejpal, C.S.; Chatterjee, N.S.; Elavarasan, K.; Lekshmi, R.G.K.; Anandan, R.; Asha, K.K.; Ravishankar, C.N. Dietary supplementation of thiamine and pyridoxine-loaded vanillic acid-grafted chitosan microspheres enhances growth performance, metabolic and immune responses in experimental rats. Int. J. Biol. Macromol. 2017, 104, 1874–1881. [Google Scholar] [CrossRef] [PubMed]
- Skulas-Ray, A.C.; Wilson, P.W.; Harris, W.S.; Brinton, E.A.; Kris-Etherton, P.M.; Richter, C.K.; Welty, F.K. Omega-3 fatty acids for the management of hypertriglyceridemia: A science advisory from the American Heart Association. Circulation 2019, 140, e673–e691. [Google Scholar] [CrossRef] [Green Version]
- O’Mahoney, L.L.; Matu, J.; Price, O.J.; Birch, K.M.; Ajjan, R.A.; Farrar, D.; Campbell, M.D. Omega-3 polyunsaturated fatty acids favourably modulate cardiometabolic biomarkers in type 2 diabetes: A meta-analysis and meta-regression of randomized controlled trials. Cardiovasc. Diabetol. 2018, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Trebatická, J.; Dukát, A.; Ďuračková, Z.; Muchová, J. Cardiovascular diseases, depression disorders and potential effects of omega-3 fatty acids. Physiol. Res. 2017, 66, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Buring, J.E. Marine n−3 fatty acids and prevention of cardiovascular disease and cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef]
- Rimm, E.B.; Appel, L.J.; Chiuve, S.E.; Djoussé, L.; Engler, M.B.; Kris-Etherton, P.M.; Lichtenstein, A.H. Seafood long-chain n-3 polyunsaturated fatty acids and cardiovascular disease: A science advisory from the American Heart Association. Circulation 2018, 138, e35–e47. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, F.B.; Manson, J.E. Marine omega-3 supplementation and cardiovascular disease: An updated meta-analysis of 13 randomized controlled trials involving 127,477 participants. J. Am. Heart Assoc. 2019, 8, e013543. [Google Scholar] [CrossRef]
- Dyerberg, J.; Bang, H.O. Haemostatic function and platelet polyunsaturated fatty acids in Eskimos. Lancet 1979, 314, 433–435. [Google Scholar] [CrossRef]
- Adili, R.; Hawley, M.; Holinstat, M. Regulation of platelet function and thrombosis by omega-3 and omega-6 polyunsaturated fatty acids. Prostagland. Other Lipid Mediat. 2018, 139, 10–18. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; OKeefe, J. Importance of maintaining a low omega-6/omega-3 ratio for reducing platelet aggregation, coagulation and thrombosis. BMJ Open Heart 2019, e001011. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Diabetes Fact Sheet No. 312; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Iwase, Y.; Kamei, N.; Takeda-Morishita, M. Antidiabetic effects of omega-3 polyunsaturated fatty acids: From mechanism to therapeutic possibilities. Pharmacol. Pharm. 2015, 6, 190. [Google Scholar] [CrossRef] [Green Version]
- Abbott, K.A.; Burrows, T.L.; Acharya, S.; Thota, R.N.; Garg, M.L. DHA-enriched fish oil reduces insulin resistance in overweight and obese adults. Prostagland. Leukot. Essent. Fat. Acids 2020, 159, 102154. [Google Scholar] [CrossRef]
- Gao, C.; Liu, Y.; Gan, Y.; Bao, W.; Peng, X.; Xing, Q.; Yang, Y. Effects of fish oil supplementation on glucose control and lipid levels among patients with type 2 diabetes mellitus: A Meta-analysis of randomized controlled trials. Lipids Health Dis. 2020, 19, 1–10. [Google Scholar] [CrossRef]
- Borsini, A.; Alboni, S.; Horowitz, M.A.; Tojo, L.M.; Cannazza, G.; Su, K.P.; Zunszain, P.A. Rescue of IL-1β-induced reduction of human neurogenesis by omega-3 fatty acids and antidepressants. Brain Behav. Immun. 2017, 65, 230–238. [Google Scholar] [CrossRef] [Green Version]
- Caughey, G.E.; James, M.J.; Proudman, S.M.; Cleland, L.G. Fish oil supplementation increases the cyclooxygenase inhibitory activity of paracetamol in rheumatoid arthritis patients. Complement. Ther. Med. 2010, 18, 171–174. [Google Scholar] [CrossRef]
- Gioxari, A.; Kaliora, A.C.; Marantidou, F.; Panagiotakos, D.P. Intake of ω-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: A systematic review and meta-analysis. Nutrition 2018, 45, 114–124. [Google Scholar] [CrossRef]
- Dinallo, V.; Marafini, I.; Di Fusco, D.; Laudisi, F.; Franzè, E.; Di Grazia, A.; Monteleone, G. Neutrophil extracellular traps sustain inflammatory signals in ulcerative colitis. J. Crohn’s Colitis 2019, 13, 772–784. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- John, S.; Luben, R.; Shrestha, S.S.; Welch, A.; Khaw, K.T.; Hart, A.R. Dietary n-3 polyunsaturated fatty acids and the aetiology of ulcerative colitis: A UK prospective cohort study. Eur. J. Gastroenterol. Hepatol. 2010, 22, 602–606. [Google Scholar] [CrossRef]
- Mozaffari, H.; Daneshzad, E.; Larijani, B.; Bellissimo, N.; Azadbakht, L. Dietary intake of fish, n-3 polyunsaturated fatty acids, and risk of inflammatory bowel disease: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2019, 1–17. [Google Scholar] [CrossRef]
- Jamshidi, A.; Cao, H.; Xiao, J.; Simal-Gandara, J. Advantages of techniques to fortify food products with the benefits of fish oil. Food Res. Int. 2020, 137, 109353. [Google Scholar] [CrossRef]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; El-Sabrout, K.; Alqaisi, O.; Dawood, M.A.; Abdelnour, S.A. Nutritional significance and health benefits of omega-3, -6 and -9 fatty acids in animals. Anim. Biotechnol. 2020, 1–13. [Google Scholar] [CrossRef]
- Dydjow-Bendek, D.; Zagozdzon, P. Total Dietary Fats, Fatty Acids, and Omega-3/Omega-6 Ratio as Risk Factors of Breast Cancer in the Polish Population—A Case-Control Study. In Vivo 2020, 34, 423–431. [Google Scholar] [CrossRef]
- Basuru, G.M.V.T.; Kariyawasam, M.G.T.R.; Alakolanga, A.G.A.W.; Abeyrathne, E.D.N.S. Development of a Simple Nontoxic Method to Extract Crude Fish Oil from Yellowfin Tuna (Thunnus albacares) Offal. In Proceedings of the International Research Conference of UWU-2019, Badula, Sri Lanka, 7–8 February 2019. [Google Scholar]
- Adeoti, I.A.; Hawboldt, K. A review of lipid extraction from fish processing by-product for use as a biofuel. Biomass Bioenergy 2014, 63, 330–340. [Google Scholar] [CrossRef]
- Rubio-Rodríguez, N.; Beltrán, S.; Jaime, I.; Sara, M.; Sanz, M.T.; Carballido, J.R. Production of omega-3 polyunsaturated fatty acid concentrates: A review. Innov. Food Sci. Emerg. Technol. 2010, 11, 1–12. [Google Scholar] [CrossRef]
- Waktola, H.D.; Zeng, A.X.; Chin, S.T.; Marriott, P.J. Advanced gas chromatography and mass spectrometry technologies for fatty acids and triacylglycerols analysis. TrAC Trends Anal. Chem. 2020, 129, 115957. [Google Scholar] [CrossRef]
- Li, J.; Vosegaard, T.; Guo, Z. Applications of nuclear magnetic resonance in lipid analyses: An emerging powerful tool for lipidomics studies. Prog. Lipid Res. 2017, 68, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Eibler, D.; Krüger, S.; Skírnisson, K.; Vetter, W. Combined thin layer chromatography and gas chromatography with mass spectrometric analysis of lipid classes and fatty acids in malnourished polar bears (Ursus maritimus) which swam to Iceland. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1046, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.A.; Leaptrot, K.L.; May, J.C.; McLean, J.A. New frontiers in lipidomics analyses using structurally selective ion mobility-mass spectrometry. TrAC Trends Anal. Chem. 2019, 116, 316–323. [Google Scholar] [CrossRef]
- Daoud, S.; Bou-Maroun, E.; Dujourdy, L.; Waschatko, G.; Billecke, N.; Cayot, P. Fast and direct analysis of oxidation levels of oil-in-water emulsions using ATR-FTIR. Food Chem. 2019, 293, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- Amaral, A.B.; Silva, M.V.D.; Lannes, S.C.D.S. Lipid oxidation in meat: Mechanisms and protective factors—A review. Food Sci. Technol. 2018, 38, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Vieira, S.A.; Zhang, G.; Decker, E.A. Biological implications of lipid oxidation products. J. Am. Oil Chem. Soc. 2017, 94, 339–351. [Google Scholar] [CrossRef]
- Johnson, D.R.; Inchingolo, R.; Decker, E.A. The ability of oxygen scavenging packaging to inhibit vitamin degradation and lipid oxidation in fish oil-in-water emulsions. Innov. Food Sci. Emerg. Technol. 2018, 47, 467–475. [Google Scholar] [CrossRef]
- Shehzad, Q.; Rehman, A.; Jafari, S.M.; Zuo, M.; Khan, M.A.; Ali, A.; Xia, W. Improving the oxidative stability of fish oil nanoemulsions by co-encapsulation with curcumin and resveratrol. Colloids Surf. B Biointerfaces 2021, 199, 111481. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.Z.; Ahmad, J.; Zafar, S.; Warsi, M.H.; Abdel-Wahab, B.A.; Akhter, S.; Alam, M.A. Omega-3 fatty acids as adjunctive therapeutics: Prospective of nanoparticles in its formulation development. Ther. Deliv. 2020, 11, 851–868. [Google Scholar] [CrossRef]
- McClements, D.J. Advances in edible nanoemulsions: Digestion, bioavailability, and potential toxicity. Prog. Lipid Res. 2020, 81, 101081. [Google Scholar] [CrossRef]
- Maki, K.C.; Dicklin, M.R. Strategies to improve bioavailability of omega-3 fatty acids from ethyl ester concentrates. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Doost, A.S.; Nasrabadi, M.N.; Kassozi, V.; Nakisozi, H.; Van der Meeren, P. Recent advances in food colloidal delivery systems for essential oils and their main components. Trends Food Sci. Technol. 2020, 99, 474–486. [Google Scholar] [CrossRef] [Green Version]
- Amoabediny, G.; Haghiralsadat, F.; Naderinezhad, S.; Helder, M.N.; Akhoundi Kharanaghi, E.; Mohammadnejad Arough, J.; Zandieh-Doulabi, B. Overview of preparation methods of polymeric and lipid-based (niosome, solid lipid, liposome) nanoparticles: A comprehensive review. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 383–400. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Walker, R.; Decker, E.A.; McClements, D.J. Development of food-grade nanoemulsions and emulsions for delivery of omega-3 fatty acids: Opportunities and obstacles in the food industry. Food Funct. 2015, 6, 41–54. [Google Scholar] [CrossRef] [Green Version]
- Kharat, M.; McClements, D.J. Fabrication and characterization of nanostructured lipid carriers (NLC) using a plant-based emulsifier: Quillaja saponin. Food Res. Int. 2019, 126, 108601. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid lipid nanoparticles and nanostructured lipid carriers: Structure, preparation and application. Adv. Pharm. Bull. 2015, 5, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.Y.; Ha, H.K.; Lee, M.R.; Kim, J.W.; Kim, H.J.; Lee, W.J. Physicochemical property and oxidative stability of whey protein concentrate multiple nanoemulsion containing fish oil. J. Food Sci. 2017, 82, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Ma, C.; Cui, F.; McClements, D.J.; Liu, X.; Liu, F. Protein-stabilized pickering emulsions: Formation, stability, properties, and applications in foods. Trends Food Sci. Technol. 2020, 103, 293–303. [Google Scholar] [CrossRef]
- Busolo, M.A.; Torres-Giner, S.; Prieto, C.; Lagaron, J.M. Electrospraying assisted by pressurized gas as an innovative high-throughput process for the microencapsulation and stabilization of docosahexaenoic acid-enriched fish oil in zein prolamine. Innov. Food Sci. Emerg. Technol. 2019, 51, 12–19. [Google Scholar] [CrossRef]
- Paul, D.; Dey, T.K.; Dhar, P. Nanoformulation and administration of PUFA-rich systems for applications in modern healthcare. In Nanostructures for Novel Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 165–200. [Google Scholar]
- Gulzar, S.; Benjakul, S. Characteristics and storage stability of nanoliposomes loaded with shrimp oil as affected by ultrasonication and microfluidization. Food Chem. 2020, 310, 125916. [Google Scholar] [CrossRef]
- Ghorbanzade, T.; Jafari, S.M.; Akhavan, S.; Hadavi, R. Nano-encapsulation of fish oil in nano-liposomes and its application in fortification of yogurt. Food Chem. 2017, 216, 146–152. [Google Scholar] [CrossRef]
- Rasti, B.; Jinap, S.; Mozafari, M.R.; Yazid, A.M. Comparative study of the oxidative and physical stability of liposomal and nanoliposomal polyunsaturated fatty acids prepared with conventional and Mozafari methods. Food Chem. 2012, 135, 2761–2770. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Sheng, Y.; Ngai, T. Pickering emulsions: Versatility of colloidal particles and recent applications. Curr. Opin. Colloid Interface Sci. 2020, 49, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.M.; Gumus, C.E.; Decker, E.A.; McClements, D.J. Improvements in the formation and stability of fish oil-in-water nanoemulsions using carrier oils: MCT, thyme oil, & lemon oil. J. Food Eng. 2017, 211, 60–68. [Google Scholar]
- Ghanbarzadeh, B.; Keivani, F.; Mohammadi, M. Encapsulation of food ingredients by solid lipid nanoparticles (SLNs). In Lipid-Based Nanostructures for Food Encapsulation Purposes; Academic Press: Cambridge, MA, USA, 2019; pp. 179–216. [Google Scholar]
- McClements, D.J. Recent developments in encapsulation and release of functional food ingredients: Delivery by design. Curr. Opin. Food Sci. 2018, 23, 80–84. [Google Scholar] [CrossRef]
- Farjami, T.; Madadlou, A. Fabrication methods of biopolymeric microgels and microgel-based hydrogels. Food Hydrocoll. 2017, 62, 262–272. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex coacervation: Principles, mechanisms and applications in microencapsulation. Int. J. Biol. Macromol. 2019, 121, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.T.; Estevinho, B.N.; Santos, L. Application of microencapsulated essential oils in cosmetic and personal healthcare products—A review. Int. J. Cosmet. Sci. 2016, 38, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Nickerson, M.T. Encapsulation of omega 3-6-9 fatty acids-rich oils using protein-based emulsions with spray drying. J. Food Sci. Technol. 2018, 55, 2850–2861. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Choi, M.J.; Ruktanonchai, U.; Min, S.G.; Chun, J.Y.; Soottitantawat, A. Physical characteristics of fish oil encapsulated by β-cyclodextrin using an aggregation method or polycaprolactone using an emulsion–diffusion method. Food Chem. 2010, 119, 1694–1703. [Google Scholar] [CrossRef]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A review of microencapsulation methods for food antioxidants: Principles, advantages, drawbacks and applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef]
- Charles, A.L.; Abdillah, A.A.; Saraswati, Y.R.; Sridhar, K.; Balderamos, C.; Masithah, E.D.; Alamsjah, M.A. Characterization of freeze-dried microencapsulation tuna fish oil with arrowroot starch and maltodextrin. Food Hydrocoll. 2021, 112, 106281. [Google Scholar] [CrossRef]
- Strobel, S.A.; Hudnall, K.; Arbaugh, B.; Cunniffe, J.C.; Scher, H.B.; Jeoh, T. Stability of fish oil in calcium alginate microcapsules cross-linked by in situ internal gelation during spray drying. Food Bioprocess. Technol. 2020, 13, 275–287. [Google Scholar] [CrossRef]
- Saari, N.H.M.; Chua, L.S. Nano-based products in beverage industry. In Nanoengineering in the Beverage Industry; Academic Press: Cambridge, MA, USA, 2019; pp. 405–436. [Google Scholar]
- Klaypradit, W.; Huang, Y.W. Fish oil encapsulation with chitosan using ultrasonic atomizer. LWT Food Sci. Technol. 2008, 41, 1133–1139. [Google Scholar] [CrossRef]
- Rusli, J.K.; Sanguansri, L.; Augustin, M.A. Stabilization of oils by microencapsulation with heated protein-glucose syrup mixtures. J. Am. Oil Chem. Soc. 2006, 83, 965–972. [Google Scholar] [CrossRef]
- Klinkesorn, U.; Sophanodora, P.; Chinachoti, P.; Decker, E.A.; McClements, D.J. Encapsulation of emulsified tuna oil in two-layered interfacial membranes prepared using electrostatic layer-by-layer deposition. Food Hydrocoll. 2005, 19, 1044–1053. [Google Scholar] [CrossRef]
- Bae, E.K.; Lee, S.J. Microencapsulation of avocado oil by spray drying using whey protein and maltodextrin. J. Microencapsul. 2008, 25, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.A.; Costa, M.J.; Rivera, M.C.; Ramos, Ó.L.; Vicente, A.A. Flavouring and coating technologies for preservation and processing of foods. In Conventional and Advanced Food Processing Technologies, 1st ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 267–311. [Google Scholar]
- Geranpour, M.; Assadpour, E.; Jafari, S.M. Recent advances in the spray drying encapsulation of essential fatty acids and functional oils. Trends Food Sci. Technol. 2020, 102, 71–90. [Google Scholar] [CrossRef]
- Boerekamp, D.M.; Andersen, M.L.; Jacobsen, C.; Chronakis, I.S.; García-Moreno, P.J. Oxygen permeability and oxidative stability of fish oil-loaded electrosprayed capsules measured by Electron Spin Resonance: Effect of dextran and glucose syrup as main encapsulating materials. Food Chem. 2019, 287, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Moreno, P.J.; Pelayo, A.; Yu, S.; Busolo, M.; Lagaron, J.M.; Chronakis, I.S.; Jacobsen, C. Physicochemical characterization and oxidative stability of fish oil-loaded electrosprayed capsules: Combined use of whey protein and carbohydrates as wall materials. J. Food Eng. 2018, 231, 42–53. [Google Scholar] [CrossRef] [Green Version]
- Fan, K.; Zhang, M.; Mujumdar, A.S. Recent developments in high efficient freeze-drying of fruits and vegetables assisted by microwave: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhong, F.; Wen, J.; McGillivray, D.; Quek, S.Y. Properties and stability of spray-dried and freeze-dried microcapsules co-encapsulated with fish oil, phytosterol esters, and limonene. Dry. Technol. 2013, 31, 707–716. [Google Scholar] [CrossRef]
- El-Messery, T.M.; Altuntas, U.; Altin, G.; Özçelik, B. The effect of spray-drying and freeze-drying on encapsulation efficiency, in vitro bioaccessibility and oxidative stability of krill oil nanoemulsion system. Food Hydrocoll. 2020, 106, 105890. [Google Scholar] [CrossRef]
- Lucía, C.; Marcela, F.; Ainhoa, L. Encapsulation of Almond Essential Oil by Co-Extrusion/Gelling Using Chitosan as Wall Material. J. Encapsul. Adsorpt. Sci. 2017, 7, 67. [Google Scholar] [CrossRef] [Green Version]
- Bamidele, O.P.; Emmambux, M.N. Encapsulation of bioactive compounds by “extrusion” technologies: A review. Crit. Rev. Food Sci. Nutr. 2020, 1–19. [Google Scholar] [CrossRef]
- Saifullah, M.; Shishir, M.R.I.; Ferdowsi, R.; Rahman, M.R.T.; Van Vuong, Q. Micro and nano encapsulation, retention and controlled release of flavor and aroma compounds: A critical review. Trends Food Sci. Technol. 2019, 86, 230251. [Google Scholar] [CrossRef]
- Kumar, L.R.; Chatterjee, N.S.; Tejpal, C.S.; Vishnu, K.V.; Anas, K.K.; Asha, K.K.; Mathew, S. Evaluation of chitosan as a wall material for microencapsulation of squalene by spray drying: Characterization and oxidative stability studies. Int. J. Biol. Macromol. 2017, 104, 1986–1995. [Google Scholar] [CrossRef]
- Vishnu, K.V.; Chatterjee, N.S.; Ajeeshkumar, K.K.; Lekshmi, R.G.K.; Tejpal, C.S.; Mathew, S.; Ravishankar, C.N. Microencapsulation of sardine oil: Application of vanillic acid grafted chitosan as a bio-functional wall material. Carbohydr. Polym. 2017, 174, 540–548. [Google Scholar] [CrossRef]
- Jinapong, N.; Suphantharika, M.; Jamnong, P. Production of instant soymilk powders by ultrafiltration, spray drying and fluidized bed agglomeration. J. Food Eng. 2008, 84, 194–205. [Google Scholar] [CrossRef]
- Ferrari, C.C.; Germer, S.P.M.; de Aguirre, J.M. Effects of spray-drying conditions on the physicochemical properties of blackberry powder. Dry. Technol. 2012, 30, 154–163. [Google Scholar] [CrossRef]
- Botrel, D.A.; Borges, S.V.; Yoshida, M.I.; de Andrade Feitosa, J.P.; de Barros Fernandes, R.V.; de Souza, H.J.B.; de Paula, R.C.M. Properties of spray-dried fish oil with different carbohydrates as carriers. J. Food Sci. Technol. 2017, 54, 4181–4188. [Google Scholar] [CrossRef]
- McClements, D.J. Encapsulation, protection, and delivery of bioactive proteins and peptides using nanoparticle and microparticle systems: A review. Adv. Colloid Interface Sci. 2018, 253, 1–22. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Qi, Y.; Zheng, L.; Wu, C.; Wang, Z.; Teng, F. Preparation and digestibility of fish oil nanoemulsions stabilized by soybean protein isolate-phosphatidylcholine. Food Hydrocoll. 2020, 100, 105310. [Google Scholar] [CrossRef]
- Klinkesorn, U.; McClements, D.J. Influence of chitosan on stability and lipase digestibility of lecithin-stabilized tuna oil-in-water emulsions. Food Chem. 2009, 114, 1308–1315. [Google Scholar] [CrossRef]
- Chang, H.W.; Tan, T.B.; Tan, P.Y.; Abas, F.; Lai, O.M.; Wang, Y.; Tan, C.P. Microencapsulation of fish oil using thiol-modified β-lactoglobulin fibrils/chitosan complex: A study on the storage stability and in vitro release. Food Hydrocoll. 2018, 80, 186–194. [Google Scholar] [CrossRef]
- Xu, X.; Sun, Q.; McClements, D.J. Effects of anionic polysaccharides on the digestion of fish oil-in-water emulsions stabilized by hydrolyzed rice glutelin. Food Res. Int. 2021, 127, 108768. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; McClements, D.J. Influence of emulsifier type on the in vitro digestion of fish oil-in-water emulsions in the presence of an anionic marine polysaccharide (fucoidan): Caseinate, whey protein, lecithin, or Tween 80. Food Hydrocoll. 2016, 61, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Gumus, C.E.; Decker, E.A.; McClements, D.J. Gastrointestinal fate of emulsion-based omega-3 oil delivery systems stabilized by plant proteins: Lentil, pea, and faba bean proteins. J. Food Eng. 2017, 207, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Qiu, C.; Zho, M.; Decker, E.A.; McClements, D.J. Influence of protein type on oxidation and digestibility of fish oil-in-water emulsions: Gliadin, caseinate, and whey protein. Food Chem. 2015, 175, 249–257. [Google Scholar] [CrossRef]



| Sl.No | Encapsulation Type | Details | Reference |
|---|---|---|---|
| 1. | Liposomes | Commonly, they contain either one or more bilayers. They therefore contain both non-polar and polar regions and so can be used to encapsulate hydrophilic and hydrophobic substances. | [63,64] |
| 2. | Solid lipid nanoparticles and nanostructured lipid carriers | Nanoemulsions are the widely acceptable methods for encapsulating the fish oils. These experiments consist of small emulsifier-coated oil droplets dispersed within water. The mean droplet diameter is below 100 nm for nanoemulsions but above this value for emulsions. | [65,66] |
| 3. | Solid lipid nanoparticles and nanostructured lipid carriers | Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) are structurally similar to nanoemulsions but the lipid phase is either fully or partially crystallized. The crystallization of the lipid phase can improve the stability of encapsulated substances by slowing down diffusion of pro-oxidants, thereby retarding their ability to interact with the omega-3 oils. | [67,68] |
| 4. | Multiple emulsions | Multiple emulsions have a more complex structure than conventional emulsions. They mainly fall into two categories depending on the relative spatial location of the different phases—water-oil-water (W/O/W) and oil-water-oil (O/W/O) emulsions. The W/O/W type is the most appropriate for the encapsulation of fish oils. | [69] |
| 5. | Microgels | Edible microgels are normally made up of small particles that are developed from food-grade proteins and/or polysaccharides. These particles contain a network of physically or chemically cross-linked biopolymer molecules. Typically, omega-3 oils would be emulsified first and then the small oil droplets would be incorporated into the microgels. | [70] |
| 6. | Nanofibers | Nanofibers consist of long thin fibrous materials that are typically assembled from food-grade biopolymers, like proteins or polysaccharides. These anofibers can sometimes be used to encapsulate and control the release of hydrophobic substances. | [71] |
| 7. | Inclusion complexes | This approach involves trapping bioactive molecules into a cyclic oligosaccharide, such as cyclodextrin, to form a molecular inclusion complex. In the case of fish oil, the non-polar tails of the fatty acids are trapped within the hydrophobic cavity formed by the cyclodextrin. | [72] |
| Encapsulant Materials | ||
|---|---|---|
| Carbohydrates | Proteins | Lipids and Waxes |
| Native starches Modified starches Resistant starches Maltodextrins Gum acacia Alginates Pectins Carrageenan Chitosan | Sodium caseinate Whey proteins Isolated whey proteins Soy proteins Gelatins Zein Albumin | Vegetable fats and oils Hydrogenated fats Palm stearin Camauba wax Bees wax Shellac Polyethylene glycol |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venugopalan, V.K.; Gopakumar, L.R.; Kumaran, A.K.; Chatterjee, N.S.; Soman, V.; Peeralil, S.; Mathew, S.; McClements, D.J.; Nagarajarao, R.C. Encapsulation and Protection of Omega-3-Rich Fish Oils Using Food-Grade Delivery Systems. Foods 2021, 10, 1566. https://doi.org/10.3390/foods10071566
Venugopalan VK, Gopakumar LR, Kumaran AK, Chatterjee NS, Soman V, Peeralil S, Mathew S, McClements DJ, Nagarajarao RC. Encapsulation and Protection of Omega-3-Rich Fish Oils Using Food-Grade Delivery Systems. Foods. 2021; 10(7):1566. https://doi.org/10.3390/foods10071566
Chicago/Turabian StyleVenugopalan, Vishnu Kalladathvalappil, Lekshmi Ramadevi Gopakumar, Ajeeshkumar Kizhakkeppurath Kumaran, Niladri Sekhar Chatterjee, Vishnuja Soman, Shaheer Peeralil, Suseela Mathew, David Julian McClements, and Ravishankar Chandragiri Nagarajarao. 2021. "Encapsulation and Protection of Omega-3-Rich Fish Oils Using Food-Grade Delivery Systems" Foods 10, no. 7: 1566. https://doi.org/10.3390/foods10071566
APA StyleVenugopalan, V. K., Gopakumar, L. R., Kumaran, A. K., Chatterjee, N. S., Soman, V., Peeralil, S., Mathew, S., McClements, D. J., & Nagarajarao, R. C. (2021). Encapsulation and Protection of Omega-3-Rich Fish Oils Using Food-Grade Delivery Systems. Foods, 10(7), 1566. https://doi.org/10.3390/foods10071566