Controlled Release of Flavor Substances from Sesame-Oil-Based Oleogels Prepared Using Biological Waxes or Monoglycerides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Oleogels
2.3. Determination of Critical Concentrations
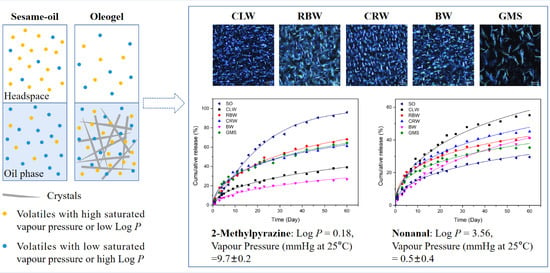
2.4. Microscopy
2.5. Texture Analysis
2.6. Thermal Properties
2.7. XRD Measurements
2.8. Determination of Cumulative Release Rate of Flavor Compounds
2.9. Data Analysis
3. Results and Discussion
3.1. Determination of Critical Concentration
3.2. Microscopic Analysis
3.3. Texture Analysis
3.4. Thermal Properties
3.5. XRD Analysis
3.6. Release Kinetics of Flavor Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wan, Y.; Li, H.; Fu, G.; Chen, X.; Chen, F.; Xie, M. The relationship of antioxidant components and antioxidant activity of sesame seed oil. J. Sci. Food Agric. 2015, 95, 2571–2578. [Google Scholar] [CrossRef] [PubMed]
- Konsoula, Z.; Liakopoulou-Kyriakides, M. Effect of endogenous antioxidants of sesame seeds and sesame oil to the thermal stability of edible vegetable oils. LWT Food Sci. Technol. 2010, 43, 1379–1386. [Google Scholar] [CrossRef]
- Kumar, C.M.; Singh, S.A. Bioactive lignans from sesame (Sesamum indicum L.): Evaluation of their antioxidant and antibacterial effects for food applications. J. Food Sci. Technol. 2015, 52, 2934–2941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varelas, C.G.; Dixon, D.G.; Steiner, C.A. Zero-order release from biphasic polymer hydrogels. J. Control. Release 1995, 34, 185–192. [Google Scholar] [CrossRef]
- Gibaldi, M.; Feldman, S. Establishment of sink conditions in dissolution rate determinations. Theoretical considerations and application to nondisintegrating dosage forms. J. Pharm. Sci. 1967, 56, 1238–1242. [Google Scholar] [CrossRef]
- Higuchi, T. Rate of Release of Medicaments from Ointment Bases Containing Drugs in Suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef]
- Neoh, T.-L.; Yoshii, H.; Furuta, T. Encapsulation and Release Characteristics of Carbon Dioxide in α-Cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2006, 56, 125–133. [Google Scholar] [CrossRef]
- Pușcaș, A.; Mureșan, V.; Socaciu, C.; Muste, S. Oleogels in Food: A Review of Current and Potential Applications. Foods 2020, 9, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, A.R. A colloidal gel perspective for understanding oleogelation. Curr. Opin. Food Sci. 2017, 15, 1–7. [Google Scholar] [CrossRef]
- Meng, Z.; Qi, K.; Guo, Y.; Wang, Y.; Liu, Y. Macro-micro structure characterization and molecular properties of emulsion-templated polysaccharide oleogels. Food Hydrocoll. 2018, 77, 17–29. [Google Scholar] [CrossRef]
- Chopin-Doroteo, M.; Morales-Rueda, J.A.; Dibildox-Alvarado, E.; Charó-Alonso, M.A.; de la Peña-Gil, A.; Toro-Vazquez, J.F. The Effect of Shearing in the Thermo-mechanical Properties of Candelilla Wax and Candelilla Wax–Tripalmitin Organogels. Food Biophys. 2011, 6, 359–376. [Google Scholar] [CrossRef]
- Troya, F.; Lerma-García, M.J.; Herrero-Martínez, J.M.; Simó-Alfonso, E.F. Classification of vegetable oils according to their botanical origin using n-alkane profiles established by GC–MS. Food Chem. 2015, 167, 36–39. [Google Scholar] [CrossRef] [PubMed]
- McGill, A.S.; Moffat, C.F.; Mackie, P.R.; Cruickshank, P. The composition and concentration of n-alkanes in retail samples of edible oils. J. Sci. Food Agric. 1993, 61, 357–362. [Google Scholar] [CrossRef]
- Moreda, W.; Pérez-Camino, M.C.; Cert, A. Gas and liquid chromatography of hydrocarbons in edible vegetable oils. J. Chromatogr. A 2001, 936, 159–171. [Google Scholar] [CrossRef]
- Giuffrè, A.M. n-Alkanes and n-Alkenes in Virgin Olive Oil from Calabria (South Italy): The Effects of Cultivar and Harvest Date. Foods 2021, 10, 290. [Google Scholar] [CrossRef]
- Giuffrè, A.M. The effect of cultivar and harvest season on the n-alkane and the n-alkene composition of virgin olive oil. Eur. Food Res. Technol. 2021, 247, 25–36. [Google Scholar] [CrossRef]
- Goh, S.H.; Gee, P.T. Noncarotenoid hydrocarbons in palm oil and palm fatty acid distillate. J. Am. Oil Chem. Soc. 1986, 63, 226–230. [Google Scholar] [CrossRef]
- Herchi, W.; Saoussem, H.; Rochut, S.; Boukhchina, S.; Kallel, H.; Pepe, C. Characterization and Quantification of the Aliphatic Hydrocarbon Fraction during Linseed Development (Linum usitatissimum L.). J. Agric. Food Chem. 2009, 57, 5832–5836. [Google Scholar] [CrossRef]
- Chen, C.H.; Terentjev, E.M. Aging and Metastability of Monoglycerides in Hydrophobic Solutions. Langmuir 2009, 25, 6717–6724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yılmaz, E.; Öğütcü, M.; Yüceer, Y.K. Physical Properties, Volatiles Compositions and Sensory Descriptions of the Aromatized Hazelnut Oil-Wax Organogels. J. Food Sci. 2015, 80, S2035–S2044. [Google Scholar] [CrossRef]
- Öğütcü, M.; Yılmaz, E.; Güneşer, O. Influence of Storage on Physicochemical and Volatile Features of Enriched and Aromatized Wax Organogels. J. Am. Oil Chem. Soc. 2015, 92, 1429–1443. [Google Scholar] [CrossRef]
- Toro-Vazquez, J.F.; Morales-Rueda, J.A.; Dibildox-Alvarado, E.; Charó-Alonso, M.; Alonzo-Macias, M.; González-Chávez, M.M. Thermal and Textural Properties of Organogels Developed by Candelilla Wax in Safflower Oil. J. Am. Oil Chem. Soc. 2007, 84, 989–1000. [Google Scholar] [CrossRef]
- Morales-Rueda, J.A.; Dibildox-Alvarado, E.; Charó-Alonso, M.A.; Weiss, R.G.; Toro-Vazquez, J.F. Thermo-mechanical properties of candelilla wax and dotriacontane organogels in safflower oil. Eur. J. Lipid Sci. Technol. 2009, 111, 207–215. [Google Scholar] [CrossRef]
- Hwang, H.-S.; Kim, S.; Singh, M.; Winkler-Moser, J.K.; Liu, S.X. Organogel Formation of Soybean Oil with Waxes. J. Am. Oil Chem. Soc. 2012, 89, 639–647. [Google Scholar] [CrossRef]
- Abdallah, D.J.; Weiss, R.G. n-Alkanes Gel n-Alkanes (and Many Other Organic Liquids). Langmuir 2000, 16, 352–355. [Google Scholar] [CrossRef]
- Maia, M.; Nunes, F.M. Authentication of beeswax (Apis mellifera) by high-temperature gas chromatography and chemometric analysis. Food Chem. 2013, 136, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Wijarnprecha, K.; Aryusuk, K.; Santiwattana, P.; Sonwai, S.; Rousseau, D. Structure and rheology of oleogels made from rice bran wax and rice bran oil. Food Res. Int. 2018, 112, 199–208. [Google Scholar] [CrossRef]
- Dassanayake, L.S.K.; Kodali, D.R.; Ueno, S.; Sato, K. Physical Properties of Rice Bran Wax in Bulk and Organogels. J. Am. Oil Chem. Soc. 2009, 86, 1163. [Google Scholar] [CrossRef]
- Doan, C.D.; Tavernier, I.; Okuro, P.K.; Dewettinck, K. Internal and external factors affecting the crystallization, gelation and applicability of wax-based oleogels in food industry. Innov. Food Sci. Emerg. Technol. 2018, 45, 42–52. [Google Scholar] [CrossRef]
- Blake, A.I.; Marangoni, A.G. Plant wax crystals display platelet-like morphology. Food Struct. 2015, 3, 30–34. [Google Scholar] [CrossRef]
- Doan, C.D.; To, C.M.; De Vrieze, M.; Lynen, F.; Danthine, S.; Brown, A.; Dewettinck, K.; Patel, A.R. Chemical profiling of the major components in natural waxes to elucidate their role in liquid oil structuring. Food Chem. 2017, 214, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, D.J.; Lu, L.; Weiss, R.G. Thermoreversible Organogels from Alkane Gelators with One Heteroatom. Chem. Mater. 1999, 11, 2907–2911. [Google Scholar] [CrossRef]
- Clarkson, C.E.; Malkin, T. 139. Alternation in long-chain compounds. Part II. An X-ray and thermal investigation of the triglycerides. J. Chem. Soc. 1934, 666–671. [Google Scholar] [CrossRef]
- Yılmaz, E.; Öǧütcü, M.; Arifoglu, N. Assessment of Thermal and Textural Characteristics and Consumer Preferences of Lemon and Strawberry Flavored Fish Oil Organogels. J. Oleo Sci. 2015, 64, 1049–1056. [Google Scholar] [CrossRef] [Green Version]
- Doan, C.D.; Tavernier, I.; Bin Sintang, M.D.; Danthine, S.; Van de Walle, D.; Rimaux, T.; Dewettinck, K. Crystallization and Gelation Behavior of Low- and High Melting Waxes in Rice Bran Oil: A Case-Study on Berry Wax and Sunflower Wax. Food Biophys. 2017, 12, 97–108. [Google Scholar] [CrossRef]
- Chen, X.-W.; Chen, Y.-J.; Wang, J.-M.; Guo, J.; Yin, S.-W.; Yang, X.-Q. Tunable volatile release from organogel-emulsions based on the self-assembly of β-sitosterol and γ-oryzanol. Food Chem. 2017, 221, 1491–1498. [Google Scholar] [CrossRef]
- Yin, W.; Washington, M.; Ma, X.; Yang, X.; Lu, A.; Shi, R.; Zhao, R.; Wang, X. Consumer acceptability and sensory profiling of sesame oils obtained from different processes. Grain Oil Sci. Technol. 2020, 3, 39–48. [Google Scholar] [CrossRef]
- Chen, X.-W.; Guo, J.; Wang, J.-M.; Yin, S.-W.; Yang, X.-Q. Controlled volatile release of structured emulsions based on phytosterols crystallization. Food Hydrocoll. 2016, 56, 170–179. [Google Scholar] [CrossRef]






| Sample | Crystallization Process | Melting Process | ||||||
|---|---|---|---|---|---|---|---|---|
| Tg (°C) | Tc1 (°C) | Tc2 (°C) | ΔHc (J/g) | Te (°C) | Tm1 (°C) | Tm2 (°C) | ΔHm (J/g) | |
| 2 wt.% CLW | ND i | ND h | ND g | ND i | ND i | ND i | ND d | ND i |
| 8 wt.% CLW | 52.73 ± 0.09 b | 41.34 ± 0.10 f | ND g | 7.68 ± 0.04 e | 58.69 ± 0.09 e | 46.89 ± 0.18 f | ND d | 7.23 ± 0.12 d |
| 5 wt.% RBW | 47.54 ± 0.25 f | 44.51 ± 0.56 e | 34.54 ± 0.19 d | 8.35 ± 0.18 d | 70.04 ± 0.72 d | 63.28 ± 0.38 d | ND d | 7.90 ± 0.11 c |
| 8 wt.% RBW | 53.52 ± 0.31 a | 49.38 ± 1.00 c | 38.28 ± 0.75 c | 12.34 ± 0.02 a | 71.26 ± 0.06 c | 65.95 ± 0.10 c | ND d | 12.11 ± 0.12 a |
| 5 wt.% CRW | 51.60 ± 0.23 c | 50.95 ± 0.27 b | 47.94 ± 0.09 b | 7.02 ± 0.03 f | 79.97 ± 0.14 b | 69.87 ± 0.35 b | 76.80 ± 0.08 a | 3.90 ± 0.05 g |
| 8 wt.% CRW | 53.71 ± 0.02 a | 53.30 ± 0.04 a | 49.81 ± 0.17 a | 11.41 ± 0.03 b | 81.26 ± 0.55 a | 71.21 ± 0.09 a | 77.72 ± 0.38 a | 6.12 ± 0.11 e |
| 3 wt.% BW | 41.31 ± 0.03 h | 38.66 ± 0.07 g | 23.28 ± 0.02 e | 1.94 ± 0.04 h | 53.00 ± 0.14 h | 45.01 ± 0.01 g | ND d | 1.76 ± 0.02 h |
| 8 wt.% BW | 48.45 ± 0.01 e | 45.59 ± 0.19 de | 34.13 ± 0.11 d | 7.02 ± 0.01 f | 54.68 ± 0.12 g | 49.76 ± 0.42 e | ND d | 4.98 ± 0.04 f |
| 5 wt.% GMS | 44.58 ± 0.03 g | 40.20 ± 0.02 f | 11.34 ± 0.08 f | 4.33 ± 0.03 g | 52.95 ± 0.05 h | 12.84 ± 0.06h | 47.18 ± 0.11 c | 4.11 ± 0.04 g |
| 8 wt.% GMS | 49.52 ± 0.04 d | 46.67 ± 0.06 d | 11.92 ± 0.10 f | 9.86 ± 0.07 c | 56.59 ± 0.10 f | 13.03 ± 0.08 h | 51.58 ± 0.25 b | 9.74 ± 0.05 b |
| Volatiles | Aroma Description | Log p | Vapor Pressure (mmHg at 25 °C) | Initial Concentration (μg/kg) | Post-Storage Concentrations (μg/kg) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CLW | RBW | CRW | BW | GMS | SO | |||||
| Significant retention effect | ||||||||||
| 2-Pentanon | ethereal, fruity | 0.9 | 38.6 ± 0.2 | 1920 ± 114 | 1985 ± 46 a | 419 ± 46 c | 345 ± 19 c | 2013 ± 76 a | 606 ± 37 b | Nd d |
| 2-Methylbutanal | flowers, grass | 1.25 | 49.3 ± 0.2 | 2151 ± 136 | 1907 ± 54 b | 662 ± 8 c | 379 ± 40 d | 2142 ± 58 a | 427 ± 79 d | Nd e |
| Pyrazine | pungent, sweet, corn, nutty | −0.28 | 19.7 ± 0.2 | 4891 ± 367 | 2787 ± 140 b | 1201 ± 56 d | 1583 ± 86 c | 3291 ± 180 a | 1376 ± 85 cd | Nd e |
| Hexanal | fatty-green, grassy | 1.97 | 10.9 ± 0.2 | 2171 ± 126 | 2144 ± 59 a | 1703 ± 15 b | 1779 ± 39 b | 2184 ± 36 a | 1702 ± 143 b | 1127 ± 68 c |
| 2-Methylthiazole | nutty, green | 1.10 | 12.9 ± 0.2 | 572 ± 19 | 451 ± 29 b | Nd d | 253 ± 20 c | 569 ± 23 a | 289 ± 19 c | Nd d |
| 4-Methylthiazole | nutty, green | 0.90 | 10.0 ± 0.2 | 3242 ± 241 | 2442 ± 137 a | 680 ± 89 b | 883 ± 40 b | 2450 ± 22 a | 930 ± 162 b | Nd c |
| 2-Methylpyrazine | nutty, cocoa-like | 0.18 | 9.7 ± 0.2 | 43,839 ± 1291 | 26,702 ± 532 b | 13,976 ± 243 d | 17,040 ± 445 c | 32,108 ± 611 a | 15,637 ± 376 c | 1718 ± 78 e |
| Furfural | almond-like | 0.73 | 2.2 ± 0.3 | 13,949 ± 67 | 7431 ± 25 b | 2148 ± 219 d | 1722 ± 28 de | 9332 ± 214 a | 4348 ± 240 c | 1356 ± 69 e |
| 2-Furanmethanol | faint, burning odor | 0.20 | 1.0 ± 0.3 | 15,654 ± 650 | 6936 ± 316 b | 3718 ± 81 c | 2583 ± 252 d | 9267 ± 282 a | 3957 ± 92 c | 1012 ± 47 e |
| 3-Methylpyridine | green | 1.19 | 6.7 ± 0.3 | 3109 ± 352 | 1616 ± 43 cd | 2107 ± 66 ab | 1839 ± 55 bc | 2322 ± 115 a | 1329 ± 130 d | 1675 ± 65 c |
| 2,4-Dimethylthiazole | meat, cocoa-like | 1.56 | 4.3 ± 0.3 | 1489 ± 47 | 947 ± 34 b | 628 ± 8 d | 721 ± 18 cd | 1354 ± 70 a | 790 ± 12 c | 275 ± 9 e |
| 1-(2-Furyl)ethanone | coffee-like | 0.52 | 0.8 ± 0.3 | 2131 ± 207 | 1279 ± 22 b | 824 ± 80 de | 1035 ± 32 cd | 1746 ± 77 a | 1187 ± 112 bc | 737 ± 15 e |
| 2,5-Dimethylpyrazine | nutty, coffee-like | 0.64 | 4.0 ± 0.2 | 37,933 ± 2490 | 24772 ± 243 b | 16,760 ± 434 d | 21,229 ± 1096 c | 33103 ± 797 a | 21,964 ± 1712 bc | 12,523 ± 541 e |
| 2-Ethylpyrazine | nutty, peanut butter | 0.71 | 4.0 ± 0.3 | 6609 ± 474 | 3791 ± 163 b | 2141 ± 33 d | 2762 ± 58 c | 4650 ± 98 a | 2898 ± 350 c | 1566 ± 85 e |
| 2,3-Dimethylpyrazine | nutty, cocoa-like | 0.64 | 3.4 ± 0.3 | 9595 ± 544 | 5026 ± 123 b | 3528 ± 192 de | 4205 ± 49 cd | 6302 ± 51 a | 4656 ± 394 bc | 3207 ± 229 e |
| 2-Vinylpyrazine | coffee-like | 0.58 | 3.3 ± 0.3 | 2822 ± 178 | 1383 ± 53 b | 960 ± 14 cd | 1118 ± 88 c | 1902 ± 37 a | 1332 ± 32 b | 914 ± 22 d |
| (5-Methyl-2-furyl)methanol | sweet caramel-like | 0.66 | 0.6 ± 0.4 | 3488 ± 129 | 564 ± 33 b | Nd c | Nd c | 912 ± 68 a | Nd c | Nd c |
| 5-Methyl-2-furaldehyde | spicy-sweet, slightly caramellic | 1.19 | 0.6 ± 0.3 | 19,949 ± 1301 | 9361 ± 482 d | 13,288 ± 311 c | 15,386 ± 224 b | 17,974 ± 593 a | 17,995 ± 264 a | 14383 ± 434 bc |
| No significant retention effect | ||||||||||
| 2-Ethyl-6-methylpyrazine | roasted baked potato | 1.17 | 2.0 ± 0.3 | 11,203 ± 492 | 5869 ± 71 bc | 5209 ± 148 c | 6547 ± 162 b | 8711 ± 161 a | 8034 ± 253 a | 8424 ± 334 a |
| 2,3,5-Trimethylpyrazine | roasted nut, baked potato | 1.10 | 1.7 ± 0.3 | 26,542 ± 1612 | 15,006 ± 169 c | 12,903 ± 275 d | 17,151 ± 109 b | 21,770 ± 555 a | 20,495 ± 870 a | 21,616 ± 754 a |
| 1H-Pyrrole-2-carbaldehyde | grassy | 0.64 | 0.1 ± 0.4 | 18,461 ± 877 | 9879 ± 34 d | 10,000 ± 567 d | 12,381 ± 459 c | 14,916 ± 454 b | 14,085 ± 219 b | 17815 ± 667 a |
| 2-Acetylpyrazine | nutty, popcorn, breadcrust | 0.16 | 0.2 ± 0.4 | 16,477 ± 398 | 8040 ± 116 d | 7261 ± 240 d | 9427 ± 119 c | 11,794 ± 405 b | 12,424 ± 231 b | 13,373 ± 287 a |
| 1-(2-Pyridinyl)ethanone | tobacco, heavy-oily-fatty | 0.87 | 0.5 ± 0.4 | 11,234 ± 701 | 5926 ± 55 d | 5122 ± 152 e | 8629 ± 209 c | 9433 ± 337 b | 9076 ± 188 bc | 11039 ± 148 a |
| 3-Ethyl-2,5-dimethylpyrazine | nutty, coffee-like | 1.63 | 1.2 ± 0.3 | 23,354 ± 1357 | 10,051 ± 435 d | 9395 ± 83 d | 12,325 ± 234 c | 14,329 ± 478 b | 15,420 ± 349 b | 18,050 ± 236 a |
| 2-Methoxyphenol | smoke, somewhat medicinal | 1.19 | 0.2 ± 0.4 | 38,612 ± 1786 | 21,634 ± 691 d | 22,497 ± 142 d | 27,067 ± 697 c | 31,870 ± 1387 b | 34,329 ± 251 b | 37,909 ± 605 a |
| Nonanal | fruity | 3.56 | 0.5 ± 0.4 | 11,488 ± 504 | 5147 ± 186 d | 6751 ± 169 c | 6293 ± 157 c | 6731 ± 212 c | 7425 ± 140 b | 8090 ± 167 a |
| 2-Acetyl-6-methylpyrazine | nutty | 0.62 | 0.1 ± 0.5 | 26,345 ± 1182 | 10,545 ± 72 d | 13,628 ± 108 c | 13,967 ± 173 c | 15,098 ± 207 b | 15,554 ± 571 b | 18,861 ± 510 a |
| 2-Methoxy-4-vinylphenol | spicy, roasted peanut | 1.93 | 0.0 ± 0.5 | 43,737 ± 1419 | 16,196 ± 162 d | 20,740 ± 518 bc | 23,432 ± 393 b | 20,378 ± 284 c | 21,890 ± 799 bc | 27,162 ± 1896 a |
| Sample | 2-Methylpyrazine | Nonanal | ||||
|---|---|---|---|---|---|---|
| k | n | R2 | k | n | R2 | |
| SO | 0.0445 | 1.1422 | 0.9966 | 0.0019 | 0.4518 | 0.9799 |
| CLW oleogel | 0.0047 | 0.5424 | 0.9877 | 0.0132 | 0.5791 | 0.9804 |
| RBW oleogel | 0.0207 | 0.6572 | 0.9898 | 0.0038 | 0.4019 | 0.9770 |
| CRW oleogel | 0.0162 | 0.5483 | 0.9820 | 0.0069 | 0.4840 | 0.9799 |
| BW oleogel | 0.0022 | 0.5402 | 0.9763 | 0.0062 | 0.6352 | 0.9897 |
| GMS oleogel | 0.0173 | 0.5759 | 0.9795 | 0.0025 | 0.3943 | 0.9750 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, M.; Cao, L.; Kang, S.; Jiang, S.; Cao, L. Controlled Release of Flavor Substances from Sesame-Oil-Based Oleogels Prepared Using Biological Waxes or Monoglycerides. Foods 2021, 10, 1828. https://doi.org/10.3390/foods10081828
Pang M, Cao L, Kang S, Jiang S, Cao L. Controlled Release of Flavor Substances from Sesame-Oil-Based Oleogels Prepared Using Biological Waxes or Monoglycerides. Foods. 2021; 10(8):1828. https://doi.org/10.3390/foods10081828
Chicago/Turabian StylePang, Min, Lulu Cao, Shengmei Kang, Shaotong Jiang, and Lili Cao. 2021. "Controlled Release of Flavor Substances from Sesame-Oil-Based Oleogels Prepared Using Biological Waxes or Monoglycerides" Foods 10, no. 8: 1828. https://doi.org/10.3390/foods10081828
APA StylePang, M., Cao, L., Kang, S., Jiang, S., & Cao, L. (2021). Controlled Release of Flavor Substances from Sesame-Oil-Based Oleogels Prepared Using Biological Waxes or Monoglycerides. Foods, 10(8), 1828. https://doi.org/10.3390/foods10081828