Beta vulgaris as a Natural Nitrate Source for Meat Products: A Review
Abstract
:1. Introduction
2. Nitrate Content in Beta vulgaris Varieties
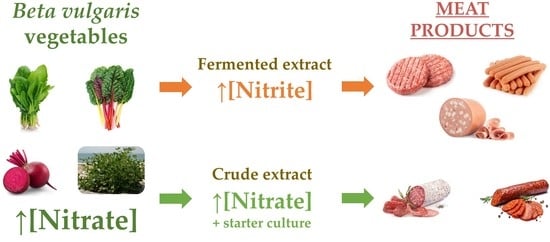
3. Application of Beta vulgaris in Meat Products
3.1. Direct Addition of Beta vulgaris Extracts in Meat Products
3.2. Fermentation of Beta vulgaris to Produce Nitrite-Rich Extracts
| Source | Meat Product | Treatments and Nitrite Content in Extracts | Sampling Point | Residual Nitrate/Nitrite | Effect | Ref. |
|---|---|---|---|---|---|---|
| Beetroot | Cooked pork sausage | 0.5% and 1.0% powder extract | Stored at 4 °C for 28 days | 4.4–5.1 ppm | Reduced L* value; no effect on b* value, texture, TBARS, sensory scores for flavor, tenderness, juiciness, and overall acceptability; increased a* value and sensory score for color | [45] |
| Beetroot and other natural extracts | Cooked pork sausage | 0.6% (1% beetroot powder in the mixed extract) | Stored at 4 °C for 4 weeks | 0.6 ppm | No effect on pH, TBARS, VBN, microbial count, sensory score for color; increased L*, a*, and b* values, shear force, and sensory scores for aroma, flavor, juiciness, chewiness, and overall acceptability | [46] |
| Beetroot and thyme essential oil | Fresh beef sausage | 1% powder extract | Stored at 4 °C for 28 days | n.e. | Reduced coagulase-positive Staphylococcus growth; no effect on sensory scores for odor, texture, and overall acceptability; increased aerobic mesophilic bacteria and sensory scores for appearance, color, and flavor | [47] |
| Beetroot | Cooked pork sausage | 3% liquid extract (fermented with Staphylococcus carnosus at 30 °C for 24 h; 748 ppm nitrite) | Final product | ~5 mg/kg | Reduced pH, a* value, residual nitrite; no effect on L* value and TPC; increased b* value, VBN, and TBARS | [50] |
| Beetroot | Cooked pork sausage | 5% and 10% liquid extract (fermented with Staphylococcus carnosus at 30 °C for 24 h; 730 ppm nitrite) | Final product | 15–30 mg/kg | Reduced pH, L*, and a* values, VBN, residual nitrite, and color scores; no effect on microbial counts, flavor, off-odor, and juiciness; increased b* value, TBARS, and overall acceptability (10%) | [51] |
| Beetroot | Low-salt frankfurters | 1%, 3%, and 5% liquid extract (fermented with Staphylococcus carnosus at 30 °C for 24 h; 729 ppm nitrite) | Refrigerated storage for 20 days | n.e. | Reduced VBN, TBARS, TPC, L*, and b* values and tenderness; no effect on sensory appearance, color, juiciness, and overall acceptance; increased pH, a* value, and flavor | [52] |
| Chard | Pork patties | 1 (with 0.006% synthetic nitrite) and 2 g powder/100 g (fermented with Staphylococcus carnosus at 37 °C for 24 h; 60,540 ppm nitrite) | Stored at 4 °C for 28 days | 21–~60 mg/kg | Reduced pH and residual nitrite; similar TBARS, curing efficiency, redness preservation, and sensory scores as controls with nitrite | [53] |
| Beetroot | Fermented and dry-cured pork sausage | 0.5% and 1% beetroot powder; Staphylococcus carnosus as starter culture | Ripening at 25 °C with RH of 95% for 1 day and decreasing 1 °C and 2% in RH every day for 6 days and at 15 °C with RH 75% for 27 days | 0–209 mg nitrate/kg; 0–7.8 mg nitrite/kg | Reduced aw, pH (1%), L*, and b* values, residual nitrate and nitrite (0.5%), and lipid oxidation; no major effect on TPC, LAB, and total coliforms; increased weight loss, a* value, and formation of nitroso pigments | [54] |
| Beetroot | Fermented and dry-cured pork sausage | 0.5% and 1% beetroot powder; Staphylococcus carnosus as starter culture | Stored at 5 °C for 60 days | 0 mg nitrate/kg; 0–4.2 mg nitrite/kg | Reduced aw, pH, L*, and b* values, residual nitrate and nitrite, and nitroso pigments; no major effect on lipid oxidation, TPC, LAB, and total coliforms; increased a* value and residual nitrite (1%) | [54] |
| Chard and beetroot | Dry-cured traditional Spanish chorizo | 6000 ppm (3000 ppm from each powder extract); Pediococcus, Staphylococcus xylosus, and Staphylococcus carnosus as starter culture | Ripening at 22 °C with 90% RH for 2 days and 14 °C with 70% RH for 23 days | n.e. | Reduced residual nitrate and nitrite, L*, a *, and b* values, hardness, and scores for redness, rancidity odor, acid flavor, rancidity flavor, and hardness; no effect on pH and protein oxidation; increased aw and sensory scores for brownness, general odor, cured odor, general flavor, cohesiveness, juiciness, and general acceptability | [55] |
| Chard and beetroot | Dry-cured traditional Spanish chorizo | 6000 ppm (3000 ppm from each powder extract); Pediococcus, Staphylococcus xylosus, and Staphylococcus carnosus as starter culture | Stored at 4 °C for 125 days | n.e. | Reduced L*, a *, and b* values and hexanal and nonanal formation; no effect on pH and protein oxidation; increased aw | [55] |
| Beetroot | Fermented beef sausage | 0.12%, 0.24%, and 0.35% powder; Staphylococcus carnosus, Pediococcus acidilactici, and Lactobacillus sakei as starter culture | Stored at 4 °C for 84 days | 1.2–3.0 mg/kg | Similar pH, residual nitrite levels, TBARS, LAB (0.12% and 0.24%), L* and b* values, texture, and sensory attributes as controls with nitrite; increased a* value | [56] |
| Beetroot with celery or spinach powder | Fermented pork sausage | 3 g/kg mixed extract; Staphylococcus carnosus, Staphylococcus xylosus, and Lactobacillus sakei as starter culture | During processing | b.d.l. | No effect on pH, LAB, aw, and sensory attributes | [57] |
3.3. Nitrate-/Nitrite-Rich Extracts from Beta vulgaris in Meat Products
4. Nitrate and Residual Nitrite Content in Meat Products
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lorenzo, J.M.; García Fontán, M.C.; Franco, I.; Carballo, J. Proteolytic and lipolytic modifications during the manufacture of dry-cured lacón, a Spanish traditional meat product: Effect of some additives. Food Chem. 2008, 110, 137–149. [Google Scholar] [CrossRef]
- Jo, K.; Lee, S.; Yong, H.I.; Choi, Y.S.; Jung, S. Nitrite sources for cured meat products. LWT 2020, 129, 109583. [Google Scholar] [CrossRef]
- Flores, M.; Toldrá, F. Chemistry, safety, and regulatory considerations in the use of nitrite and nitrate from natural origin in meat products. Meat Sci. 2021, 171, 108272. [Google Scholar] [CrossRef]
- Alahakoon, A.U.; Jayasena, D.D.; Ramachandra, S.; Jo, C. Alternatives to nitrite in processed meat: Up to date. Trends Food Sci. Technol. 2015, 45, 37–49. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Sineiro, J.; Amado, I.R.; Franco, D. Influence of natural extracts on the shelf life of modified atmosphere-packaged pork patties. Meat Sci. 2014, 96, 526–534. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.; Kim, S.; Lee, J.; Ha, J.; Choi, Y.; Oh, H.; Choi, K.H.; Yoon, Y. Microbiological safety of processed meat products formulated with low nitrite concentration—A review. Asian Australas. J. Anim. Sci. 2018, 31, 1073–1077. [Google Scholar] [CrossRef]
- Asioli, D.; Aschemann-Witzel, J.; Caputo, V.; Vecchio, R.; Annunziata, A.; Næs, T.; Varela, P. Making sense of the “clean label” trends: A review of consumer food choice behavior and discussion of industry implications. Food Res. Int. 2017, 99, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Dickson-Spillmann, M.; Siegrist, M.; Keller, C. Attitudes toward chemicals are associated with preference for natural food. Food Qual. Prefer. 2011, 22, 149–156. [Google Scholar] [CrossRef]
- Agregán, R.; Franco, D.; Carballo, J.; Tomasevic, I.; Barba, F.J.; Gómez, B.; Muchenje, V.; Lorenzo, J.M. Shelf life study of healthy pork liver pâté with added seaweed extracts from Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Food Res. Int. 2018, 112, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Nikmaram, N.; Budaraju, S.; Barba, F.J.; Lorenzo, J.M.; Cox, R.B.; Mallikarjunan, K.; Roohinejad, S. Application of plant extracts to improve the shelf-life, nutritional and health-related properties of ready-to-eat meat products. Meat Sci. 2018, 145, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Gullón, P.; Pateiro, M.; Munekata, P.E.S.; Zhang, W.; Lorenzo, J.M. Tomato as potential source of natural additives for meat industry. A review. Antioxidants 2020, 9, 73. [Google Scholar] [CrossRef] [Green Version]
- Pateiro, M.; Barba, F.J.; Domínguez, R.; Sant’Ana, A.S.; Mousavi Khaneghah, A.; Gavahian, M.; Gómez, B.; Lorenzo, J.M. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef]
- Maruyama, S.; Streletskaya, N.A.; Lim, J. Clean label: Why this ingredient but not that one? Food Qual. Prefer. 2021, 87, 104062. [Google Scholar] [CrossRef]
- Aschemann-Witzel, J.; Varela, P.; Peschel, A.O. Consumers’ categorization of food ingredients: Do consumers perceive them as ‘clean label’ producers expect? An exploration with projective mapping. Food Qual. Prefer. 2019, 71, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Munekata, P.E.S.; Pateiro, M.; Domínguez, R.; Santos, E.M.; Lorenzo, J.M. Cruciferous vegetables as sources of nitrate in meat products. Curr. Opin. Biotechnol. 2021, 38, 1–7. [Google Scholar]
- Pateiro, M.; Gómez-Salazar, J.A.; Jaime-Patlán, M.; Sosa-Morales, M.E.; Lorenzo, J.M. Plant extracts obtained with green solvents as natural antioxidants in fresh meat products. Antioxidants 2021, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Lange, W.; Brandenburg, W.A.; de Bock, T.S.M. Taxonomy and cultonomy of beet (Beta vulgaris L.). Bot. J. Linn. Soc. 1999, 130, 81–96. [Google Scholar] [CrossRef]
- Barlow, S. Beta Species. Available online: http://www.plantnames.unimelb.edu.au/Sorting/Beta.html (accessed on 11 June 2021).
- Maucieri, C.; Nicoletto, C.; Zanin, G.; Xiccato, G.; Borin, M.; Sambo, P. Composition and quality traits of vegetables grown in a low-tech aquaponic system at different fish stocking densities. J. Sci. Food Agric. 2020, 100, 4310–4318. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Assaha, D.V.M.; Islam, M.S.; Hassan, M.M.; Sabagh, A.E.; Saneoka, H. NaCl enhance the growth of swiss chard (Beta vulgaris L.) leaves under potassium-deficient conditions. J. Soil Sci. Plant Nutr. 2021, 1–8. [Google Scholar] [CrossRef]
- Quijano, L.; Yusà, V.; Font, G.; McAllister, C.; Torres, C.; Pardo, O. Risk assessment and monitoring programme of nitrates through vegetables in the region of Valencia (Spain). Food Chem. Toxicol. 2017, 100, 42–49. [Google Scholar] [CrossRef] [PubMed]
- León, V.M.; Luzardo, O.P.; Martín León, V.; Luzardo, O.P. Evaluation of nitrate contents in regulated and non-regulated leafy vegetables of high consumption in the Canary Islands, Spain: Risk assessment. Food Chem. Toxicol. 2020, 146, 111812. [Google Scholar] [CrossRef]
- Brkić, D.; Bošnir, J.; Bevardi, M.; Bošković, A.G.; Miloš, S.; Lasić, D.; Krivohlavek, A.; Racz, A.; Ćuić, A.M.; Trstenjak, N.U. Nitrate in Leafy Green Vegetables and Estimated Intake. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 31–41. [Google Scholar] [CrossRef]
- Libutti, A.; Trotta, V.; Rivelli, A.R. Biochar, vermicompost, and compost as soil organic amendments: Influence on Growth Parameters, Nitrate and Chlorophyll Content of Swiss Chard (Beta vulgaris L. var. cycla). Agronomy 2020, 10, 346. [Google Scholar] [CrossRef] [Green Version]
- Libutti, A.; Rivelli, A.R. Quanti-qualitative response of swiss chard (Beta vulgaris L. var. cycla) to soil amendment with biochar-compost mixtures. Agronomy 2021, 11, 307. [Google Scholar] [CrossRef]
- Kaburagi, E.; Yamada, M.; Baba, T.; Fujiyama, H.; Murillo-Amador, B.; Yamada, S. Aquaponics using saline groundwater: Effect of adding microelements to fish wastewater on the growth of Swiss chard (Beta vulgaris L. spp. cicla). Agric. Water Manag. 2020, 227, 105851. [Google Scholar] [CrossRef]
- Pérez-Urrestarazu, L.; Lobillo-Eguíba, J.; Fernández-Cañero, R.; Fernández-Cabanás, V.M. Food safety concerns in urban aquaponic production: Nitrate contents in leafy vegetables. Urban For. Urban Green. 2019, 44, 126431. [Google Scholar] [CrossRef]
- Bulgari, R.; Baldi, A.; Ferrante, A.; Lenzi, A. Yield and quality of basil, Swiss chard, and rocket microgreens grown in a hydroponic system. N. Z. J. Crop Hortic. Sci. 2017, 45, 119–129. [Google Scholar] [CrossRef]
- Menal-Puey, S.; Asensio, E. Validation of a rapid method for detecting nitrate in chard (Beta vulgaris cycla). Analysis of Spanish commercial samples marketed in the region of Huesca, Spain, and estimation of the daily intake. Rev. Esp. Nutr. Hum. Diet. 2015, 19, 4–11. [Google Scholar] [CrossRef] [Green Version]
- Kołota, E.; Adamczewska-Sowińska, K.; Balbierz, A. Response of swiss chard (Beta vulgaris L. var. cicla L.) to nitrogen fertilization. Acta Sci. Pol. Hortorum Cultus 2017, 16, 47–56. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Soteriou, G.A.; Colla, G.; Rouphael, Y. The occurrence of nitrate and nitrite in Mediterranean fresh salad vegetables and its modulation by preharvest practices and postharvest conditions. Food Chem. 2019, 285, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Miceli, A.; Miceli, C. Effect of nitrogen fertilization on the quality of swiss chard at harvest and during storage as minimally processed produce. J. Food Qual. 2014, 37, 125–134. [Google Scholar] [CrossRef]
- Corradini, F.; Correa, A.; Moyano, M.S.; Sepúlveda, P.; Quiroz, C. Nitrate, arsenic, cadmium, and lead concentrations in leafy vegetables: Expected average values for productive regions of Chile. Arch. Agron. Soil Sci. 2018, 64, 299–317. [Google Scholar] [CrossRef]
- Ugrinović, M.K. Contents of oxalic acid, nitrate and reduced nitrogen in different parts of beetroot (Beta vulgaris var. conditiva Alef.) at different rates of nitrogen fertilization. Afr. J. Agric. Res. 2012, 7, 3066–3072. [Google Scholar] [CrossRef]
- Dos Santos Baião, D.; Conte-Junior, C.A.; Paschoalin, V.M.F.; Alvares, T.S. Quantitative and Comparative Contents of Nitrate and Nitrite in Beta vulgaris L. by Reversed-Phase High-Performance Liquid Chromatography-Fluorescence. Food Anal. Methods 2016, 9, 1002–1008. [Google Scholar] [CrossRef] [Green Version]
- Croitoru, M.D.; Fülöp, I.; Miklos, A.; Hosszú, B.; Tătar, V.L.; Muntean, D.L. Presence of nitrate and nitrite in vegetables grown for self-consumption. Farmacia 2015, 63, 530–533. [Google Scholar]
- Rubóczki, T.; Raczkó, V.; Takácsné Hájos, M. Evaluation of morphological parameters and bioactive compounds in different varieties of beetroot (Beta vulgaris L. ssp. esculenta GURKE var. rubra L.). Int. J. Hortic. Sci. 2015, 21, 31–35. [Google Scholar] [CrossRef]
- Wruss, J.; Waldenberger, G.; Huemer, S.; Uygun, P.; Lanzerstorfer, P.; Müller, U.; Höglinger, O.; Weghuber, J. Compositional characteristics of commercial beetroot products and beetroot juice prepared from seven beetroot varieties grown in Upper Austria. J. Food Compos. Anal. 2015, 42, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Hamid, M.G.; Mohamed Nour, A.A.A. Effect of different drying methods on quality attributes of beetroot (Beta vulgaris) slices. World J. Sci. Technol. Sustain. Dev. 2018, 15, 287–298. [Google Scholar] [CrossRef]
- Maroušek, J.; Kolář, L.; Vochozka, M.; Stehel, V.; Maroušková, A. Novel method for cultivating beetroot reduces nitrate content. J. Clean. Prod. 2017, 168, 60–62. [Google Scholar] [CrossRef]
- Jabeen, A.; Narayan, S.; Hussain, K.; Ahmed Mir, S.; Khan, F.A. Effect of Organic Manures and Biofertilizers on Quality of Spinach Beet (Beta vulgaris var. bengalensis). Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1312–1317. [Google Scholar] [CrossRef]
- Thampi, S.S.; Vethamoni, P.I. Quality improvement of palak (Beta vulgaris var. bengalensis Hort.) through organic manures. J. Pharmacogn. Phytochem. 2019, 8, 938–942. [Google Scholar]
- Boari, F.; Cefola, M.; Di Gioia, F.; Pace, B.; Serio, F.; Cantore, V. Effect of cooking methods on antioxidant activity and nitrate content of selected wild Mediterranean plants. Int. J. Food Sci. Nutr. 2013, 64, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Kim, H.J.; Kyriacou, M.C.; Rouphael, Y. Nitrate in fruits and vegetables. Sci. Hortic. 2018, 237, 221–238. [Google Scholar] [CrossRef]
- Jin, S.K.; Choi, J.S.; Moon, S.S.; Jeong, J.Y.; Kim, G.D. The assessment of red beet as a natural colorant, and evaluation of quality properties of emulsified pork sausage containing red beet powder during cold storage. Korean J. Food Sci. Anim. Resour. 2014, 34, 472–481. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.K.; Choi, J.S.; Yang, H.S.; Park, T.S.; Yim, D.G. Natural curing agents as nitrite alternatives and their effects on the physicochemical, microbiological properties and sensory evaluation of sausages during storage. Meat Sci. 2018, 146, 34–40. [Google Scholar] [CrossRef]
- Lages, L.Z.; Radünz, M.; Gonçalves, B.T.; Silva da Rosa, R.; Fouchy, M.V.; de Cássia dos Santos da Conceição, R.; Gularte, M.A.; Barboza Mendonça, C.R.; Gandra, E.A. Microbiological and sensory evaluation of meat sausage using thyme (Thymus vulgaris, L.) essential oil and powdered beet juice (Beta vulgaris L., Early Wonder cultivar). LWT 2021, 148, 111794. [Google Scholar] [CrossRef]
- Gøtterup, J.; Olsen, K.; Knöchel, S.; Tjener, K.; Stahnke, L.H.; Møller, J.K.S. Relationship between nitrate/nitrite reductase activities in meat associated staphylococci and nitrosylmyoglobin formation in a cured meat model system. Int. J. Food Microbiol. 2007, 120, 303–310. [Google Scholar] [CrossRef]
- Hammes, W.P. Metabolism of nitrate in fermented meats: The characteristic feature of a specific group of fermented foods. Food Microbiol. 2012, 29, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.-E.; Kim, T.-K.; Kim, H.-W.; Seo, D.-H.; Kim, Y.-B.; Jeon, K.-H.; Choi, Y.-S. Effect of natural pre-converted nitrite sources on color development in raw and cooked pork sausage. Asian Australas. J. Anim. Sci. 2018, 31, 1358–1365. [Google Scholar] [CrossRef]
- Choi, Y.-S.; Kim, T.-K.; Jeon, K.-H.; Park, J.-D.; Kim, H.-W.; Hwang, K.-E.; Kim, Y.-B. Effects of pre-converted nitrite from red beet and ascorbic acid on quality characteristics in meat emulsions. Korean J. Food Sci. Anim. Resour. 2017, 37, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Hwang, K.-E.; Kim, T.-K.; Kim, H.-W.; Oh, N.-S.; Kim, Y.-B.; Jeon, K.-H.; Choi, Y.-S. Effect of fermented red beet extracts on the shelf stability of low-salt frankfurters. Food Sci. Biotechnol. 2017, 26, 929–936. [Google Scholar] [CrossRef]
- Shin, D.-M.; Hwang, K.-E.; Lee, C.-W.; Kim, T.-K.; Park, Y.-S.; Han, S.G. Effect of Swiss Chard (Beta vulgaris var. cicla) as nitrite replacement on color stability and shelf-life of cooked pork patties during refrigerated storage. Korean J. Food Sci. Anim. Resour. 2017, 37, 418–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozaki, M.M.; Munekata, P.E.S.; Jacinto-Valderrama, R.A.; Efraim, P.; Pateiro, M.; Lorenzo, J.M.; Pollonio, M.A.R. Beetroot and radish powders as natural nitrite source for fermented dry sausages. Meat Sci. 2021, 171, 108275. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Zamora, L.; Peñalver, R.; Ros, G.; Nieto, G. Substitution of synthetic nitrates and antioxidants by spices, fruits and vegetables in clean label Spanish chorizo. Food Res. Int. 2021, 139, 109835. [Google Scholar] [CrossRef] [PubMed]
- Sucu, C.; Turp, G.Y. The investigation of the use of beetroot powder in Turkish fermented beef sausage (sucuk) as nitrite alternative. Meat Sci. 2018, 140, 158–166. [Google Scholar] [CrossRef]
- Pennisi, L.; Verrocchi, E.; Paludi, D.; Vergara, A. Effects of vegetable powders as nitrite alternative in Italian dry fermented sausage. Ital. J. Food Saf. 2020, 9, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Wu, L.; Guan, W. Dietary nitrates, nitrites, and nitrosamines intake and the risk of gastric cancer: A meta-analysis. Nutrients 2015, 7, 9872–9895. [Google Scholar] [CrossRef]
- De Mey, E.; De Maere, H.; Paelinck, H.; Fraeye, I. Volatile N-nitrosamines in meat products: Potential precursors, influence of processing, and mitigation strategies. Crit. Rev. Food Sci. Nutr. 2017, 57, 2909–2923. [Google Scholar] [CrossRef]

| Scientific Name | Common Name | Plant Part (Nitrate Content in FW) | Class 1 | Ref. |
|---|---|---|---|---|
| Beta vulgaris subsp. vulgaris var. cicla | Chard | Leaf (163–361 mg/kg) | VL–L | [25] |
| Beta vulgaris subsp. vulgaris var. cicla | Chard | Leaf (163–333 mg/kg) | VL–L | [24] |
| Beta vulgaris subsp. vulgaris var. cicla | Chard | Leaf (20–2820 mg/kg) | VL–VH | [23] |
| Beta vulgaris subsp. vulgaris var. cicla | Chard | Leaf (0–4362 mg/kg) | VL–VH | [22] |
| Beta vulgaris subsp. vulgaris var. cicla | Chard | Leaf (0–3509 mg/kg) | VL–VH | [21] |
| Beta vulgaris subsp. vulgaris var. cicla | Chard | Leaf (143–3050 mg/kg) | VL–VH | [33] |
| Beta vulgaris subsp. vulgaris var. cicla | Chard | Leaf (591–3571 mg/kg) | L–VH | [32] |
| Beta vulgaris subsp. vulgaris var. cicla | Chard | Leaf (261–5568 mg/kg) | L–EH | [31] |
| Beta vulgaris subsp. vulgaris var. cicla | Chard | Blade (353–662 mg/kg) | L–M | [30] |
| Beta vulgaris subsp. vulgaris var. cicla | Chard | Petiole (670–1022 mg/kg) | M–H | [30] |
| Beta vulgaris subsp. vulgaris var. cicla | Chard | Leaf (967–9093 mg/kg) | M–EH | [29] |
| Beta vulgaris subsp. vulgaris var. cicla | Chard | Leaf (1061 mg/kg) | H | [28] |
| Beta vulgaris subsp. vulgaris var. cicla | Chard | Leaf (2400 mg/kg) | H | [27] |
| Beta vulgaris L. spp. cicla cv. Seiyou Shirokuki | Chard | Leaf (1000–3000 mg/kg) | H–VH | [26] |
| Beta vulgaris subsp. vulgaris var. cicla | Chard | Leaf (1400–3400 mg/kg) | H–VH | [20] |
| Beta vulgaris subsp. vulgaris var. cicla | Chard | Leaf (3490–5912 mg/kg) | VH–EH | [19] |
| Beta vulgaris subsp. vulgaris var. conditiva Alef. | Beetroot | Leaf lamina (8–156 mg/kg) | VL | [34] |
| Beta vulgaris subsp. vulgaris var. vulgaris | Beetroot | Root (101–552 mg/kg) | VL–L | [35] |
| Beta vulgaris subsp. vulgaris var. vulgaris | Beetroot | Root (39–601 mg/kg) | VL–M | [36] |
| Beta vulgaris subsp. vulgaris var. conditiva Alef. | Beetroot | Leaf petiole (204–2496 mg/kg) | VL–VH | [34] |
| Beta vulgaris L. ssp. esculenta GURKE var. rubra L. | Beetroot | Root (700–850 mg/kg) | M | [37] |
| Beta vulgaris subsp. vulgaris var. conditiva alef. | Beetroot | Root (555–2896 mg/kg) | M–VH | [34] |
| Beta vulgaris subsp. vulgaris var. conditiva | Beetroot | Root (564–4626 mg/kg) | M–VH | [38] |
| Beta vulgaris subsp. vulgaris var. vulgaris | Beetroot | Root (1977 mg/kg) | H | [39] |
| Beta vulgaris L. subsp. vulgaris var. conditiva alef., mid–late variety, intensely purple, spherical, napiform | Beetroot | Root (2320 mg/kg) | H | [40] |
| Beta vulgaris var. bengalensis | Spinach beet | Leaf (268–811 mg/kg) | L–M | [41] |
| Beta vulgaris var. bengalensis | Spinach beet | Leaf (1801–2136 mg/kg) | H | [42] |
| Beta vulgaris (L.) subsp. maritima (L.) Arcang. | Sea beet | Leaves + young stems (673 mg/kg) | M | [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munekata, P.E.S.; Pateiro, M.; Domínguez, R.; Pollonio, M.A.R.; Sepúlveda, N.; Andres, S.C.; Reyes, J.; Santos, E.M.; Lorenzo, J.M. Beta vulgaris as a Natural Nitrate Source for Meat Products: A Review. Foods 2021, 10, 2094. https://doi.org/10.3390/foods10092094
Munekata PES, Pateiro M, Domínguez R, Pollonio MAR, Sepúlveda N, Andres SC, Reyes J, Santos EM, Lorenzo JM. Beta vulgaris as a Natural Nitrate Source for Meat Products: A Review. Foods. 2021; 10(9):2094. https://doi.org/10.3390/foods10092094
Chicago/Turabian StyleMunekata, Paulo E. S., Mirian Pateiro, Rubén Domínguez, Marise A. R. Pollonio, Néstor Sepúlveda, Silvina Cecilia Andres, Jorge Reyes, Eva María Santos, and José M. Lorenzo. 2021. "Beta vulgaris as a Natural Nitrate Source for Meat Products: A Review" Foods 10, no. 9: 2094. https://doi.org/10.3390/foods10092094
APA StyleMunekata, P. E. S., Pateiro, M., Domínguez, R., Pollonio, M. A. R., Sepúlveda, N., Andres, S. C., Reyes, J., Santos, E. M., & Lorenzo, J. M. (2021). Beta vulgaris as a Natural Nitrate Source for Meat Products: A Review. Foods, 10(9), 2094. https://doi.org/10.3390/foods10092094