Current Advances on the Development and Application of Probiotic-Loaded Edible Films and Coatings for the Bioprotection of Fresh and Minimally Processed Fruit and Vegetables
Abstract
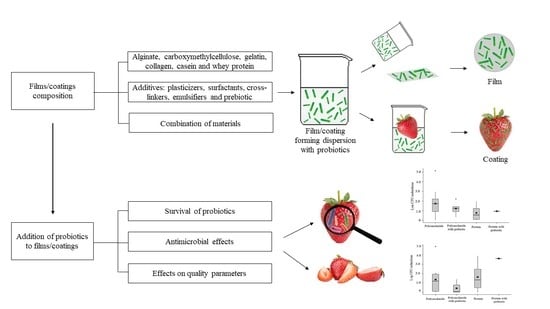
:1. Introduction
2. Materials Used to Formulate Probiotic-Loaded Edible Films/Coatings for Application to Fresh and Minimally Processed Fruit and Vegetables
3. Probiotics Loaded into Edible Films/Coatings for Application to Fresh and Minimally Processed Fruit and Vegetables
Survival of Probiotics in Edible Films/Coatings
4. Antimicrobial Effects of Probiotic-Loaded Edible Films/Coatings Applied to Fresh and Minimally Processed Fruit and Vegetables
5. Effects of Probiotic-Loaded Edible Films/Coatings on Quality Parameters of Fresh and Minimally Processed Fruit and Vegetables
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R. Antimicrobial food packaging based on sustainable Bio-based materials for reducing foodborne pathogens: A review. Food Chem. 2020, 310, 125915. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.M.B.; Jafarpour, D. Bioactive edible film based on Konjac glucomannan and probiotic Lactobacillus plantarum strains: Physicochemical properties and shelf life of fresh-cut kiwis. J. Food Sci. 2021, 86, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Linares-Morales, J.R.; Gutiérrez-Méndez, N.; Rivera-Chavira, B.E.; Pérez-Vega, S.B.; Nevárez-Moorillón, G.V. Biocontrol processes in fruits and fresh produce, the use of lactic acid bacteria as a sustainable option. Front. Sustain. Food Syst. 2018, 2, 50. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Iqbal, N. Post-harvest pathogens and disease management of horticultural crop: A brief review. Plant Arch. 2020, 20, 2054–2058. [Google Scholar]
- Papoutsis, K.; Mathioudakis, M.M.; Hasperué, J.H.; Ziogas, V. Non-chemical treatments for preventing the postharvest fungal rotting of citrus caused by Penicillium digitatum (green mold) and Penicillium italicum (blue mold). Trends Food Sci. Technol. 2019, 86, 479–491. [Google Scholar] [CrossRef]
- Khalil, O.A.; Mounir, A.M.; Hassanien, R.A. Effect of gamma irradiated Lactobacillus bacteria as an edible coating on enhancing the storage of tomato under cold storage conditions. J. Radiat. Res. Appl. Sci. 2020, 13, 318–330. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, M.B.; Echeverría, G.; Viñas, I.; López, M.L.; Abadias, M. Biopreservation of fresh-cut pear using Lactobacillus rhamnosus GG and effect on quality and volatile compounds. LWT–Food Sci. Technol. 2018, 87, 581–588. [Google Scholar] [CrossRef] [Green Version]
- Zudaire, L.; Viñas, I.; Plaza, L.; Iglesias, M.B.; Abadias, M.; Aguiló-Aguayo, I. Evaluation of postharvest calcium treatment and biopreservation with Lactobacillus rhamnosus GG on the quality of fresh-cut ‘Conference’ pears. J. Sci. Food Agric. 2018, 98, 4978–4987. [Google Scholar] [CrossRef] [Green Version]
- Massoud, R.; Khodaeii, D.; Hamidi-Esfahani, Z.; Khosravi-Darani, K. The effect of edible probiotic coating on quality of fresh fruits and vegetables: Fresh strawberries as a case study. Biomass Convers. Biorefinery 2021, 1–10. [Google Scholar] [CrossRef]
- Guimarães, A.; Abrunhosa, L.; Pastrana, L.M.; Cerqueira, M.A. Edible films and coatings as carriers of living microorganisms: A new strategy towards biopreservation and healthier foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 594–614. [Google Scholar] [CrossRef] [Green Version]
- Pop, O.L.; Pop, C.R.; Dufrechou, M.; Vodnar, D.C.; Socaci, S.A.; Dulf, F.V.; Minervini, F.; Suharoschi, R. Edible films and coatings functionalization by probiotic incorporation: A review. Polymers 2020, 12, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temiz, N.N.; Özdemir, K.S. Microbiological and physicochemical quality of strawberries (Fragaria × ananassa) coated with Lactobacillus rhamnosus and inulin enriched gelatin films. Postharvest Biol. Technol. 2021, 173, 111433. [Google Scholar] [CrossRef]
- Pandhi, S.; Kumar, A.; Alam, T. Probiotic edible films and coatings: Concerns, applications and future prospects. J. Packag. Technol. Res. 2019, 3, 261–268. [Google Scholar] [CrossRef]
- Campaniello, D.; Bevilacqua, A.; Speranza, B.; Sinigaglia, M.; Corbo, M.R. Alginate-and gelatin-coated apple pieces as carriers for Bifidobacterium animalis subsp. lactis DSM 10140. Front. Microbiol. 2020, 11, 566596. [Google Scholar] [CrossRef] [PubMed]
- Pavli, F.; Tassou, C.; Nychas, G.J.E.; Chorianopoulos, N. Probiotic incorporation in edible films and coatings: Bioactive solution for functional foods. Int. J. Mol. Sci. 2018, 19, 150. [Google Scholar] [CrossRef] [Green Version]
- Espitia, P.J.; Batista, R.A.; Azeredo, H.M.; Otoni, C.G. Probiotics and their potential applications in active edible films and coatings. Food Res. J. 2016, 90, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Tapia, M.S.; Rojas-Graü, M.A.; Rodríguez, F.J.; Ramírez, J.; Carmona, A.; Martin-Belloso, O. Alginate-and gellan-based edible films for probiotic coatings on fresh-cut fruits. J. Food Sci. 2007, 72, E190–E196. [Google Scholar] [CrossRef]
- Speranza, B.; Campaniello, D.; Bevilacqua, A.; Altieri, C.; Sinigaglia, M.; Corbo, M.R. Viability of Lactobacillus plantarum on fresh-cut chitosan and alginate-coated apple and melon pieces. Front. Microbiol. 2018, 9, 2538. [Google Scholar] [CrossRef] [Green Version]
- Zoghi, A.; Khosravi-Darani, K.; Mohammadi, R. Application of edible films containing probiotics in food products. J. Consum. Prot. Food Saf. 2020, 15, 307–320. [Google Scholar] [CrossRef]
- Rodrigues, F.J.; Cedran, M.F.; Garcia, S. Influence of linseed mucilage incorporated into an alginate-base edible coating containing probiotic bacteria on shelf-life of fresh-cut yacon (Smallanthus sonchifolius). Food Bioprocess Technol. 2018, 11, 1605–1614. [Google Scholar] [CrossRef]
- Shigematsu, E.; Dorta, C.; Rodrigues, F.J.; Cedran, M.F.; Giannoni, J.A.; Oshiiwa, M.; Mauro, M.A. Edible coating with probiotic as a quality factor for minimally processed carrots. J. Food Sci. Technol. 2018, 55, 3712–3720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bambace, M.F.; Alvarez, M.V.; Del Rosario Moreira, M. Novel functional blueberries: Fructo-oligosaccharides and probiotic lactobacilli incorporated into alginate edible coatings. Int. Food Res. J. 2019, 122, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Dianin, I.B.; Jr, A.G.O.; Pimentel, T.C.; Hernandes, N.F.; Costa, G.N. Edible biofilms formulated with whey protein isolate and L. casei probiotic culture: Characterization and application in tomatoes and grapes. Chem. Eng. Trans. 2019, 75, 469–474. [Google Scholar] [CrossRef]
- Elabd, M.A. Development of bioactive packaging: Probiotic coating for improving fresh-cut apples quality. Int. J. Tech. Res. Sci. 2019, 4, 17–24. [Google Scholar] [CrossRef]
- Khodaei, D.; Hamidi-Esfahani, Z. Influence of bioactive edible coatings loaded with Lactobacillus plantarum on physicochemical properties of fresh strawberries. Postharvest Biol. Technol. 2019, 156, 110944. [Google Scholar] [CrossRef]
- Marín, A.; Plotto, A.; Atarés, L.; Chiralt, A. Lactic acid bacteria incorporated into edible coatings to control fungal growth and maintain postharvest quality of grapes. HortScience 2019, 54, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Shigematsu, E.; Dorta, C.; Santos, D.N.; Ferreira, K.A.; Góes-Favoni, S.P.; Oshiiwa, M.; Mauro, M.A. Edible coating with coconut water to preserve probiotic strains and sensory characteristics of minimally processed carrots. Int. Food Res. J. 2019, 26, 1285–1292. [Google Scholar]
- Li, S.; Ma, Y.; Ji, T.; Sameen, D.E.; Ahmed, S.; Qin, W.; Dai, J.; Li, S.; Liu, Y. Cassava starch/carboxymethylcellulose edible films embedded with lactic acid bacteria to extend the shelf life of banana. Carbohydr. Polym. 2020, 248, 116805. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.V.; Bambace, M.F.; Quintana, G.; Gomez-Zavaglia, A.; del Rosario Moreira, M. Prebiotic-alginate edible coating on fresh-cut apple as a new carrier for probiotic lactobacilli and bifidobacterial. LWT-Food Sci. Technol. 2021, 137, 110483. [Google Scholar] [CrossRef]
- Senturk Parreidt, T.; Müller, K.; Schmid, M. Alginate-based edible films and coatings for food packaging applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soukoulis, C.; Singh, P.; Macnaughtan, W.; Parmenter, C.; Fisk, I.D. Compositional and physicochemical factors governing the viability of Lactobacillus rhamnosus GG embedded in starch-protein based edible films. Food Hydrocoll. 2016, 52, 876–887. [Google Scholar] [CrossRef]
- Arnon-Rips, H.; Poverenov, E. Improving food products’ quality and storability by using layer by layer edible coatings. Trends Food Sci. Technol. 2018, 75, 81–92. [Google Scholar] [CrossRef]
- Moradi, M.; Guimarães, J.T.; Sahin, S. Current applications of exopolysaccharides from lactic acid bacteria in the development of food active edible packaging. Curr. Opin. Food Sci. 2021, 40, 33–39. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of lactic acid bacteria: Structure, bioactivity and associations: A review. Carbohydr. Polym. 2019, 207, 317–332. [Google Scholar] [CrossRef]
- Pérez Sira, E.; Dufour, D. Native and modified starches as matrix for edible films and covers. Nutr. Food Sci. Int. J. 2017, 3, 555–615. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO). Guidelines for the Evaluation of Probiotics in Food. Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; WHO: London, ON, Canada, 2002. [Google Scholar]
- Agriopoulou, S.; Stamatelopoulou, E.; Sachadyn-Król, M.; Varzakas, T. Lactic acid bacteria as antibacterial agents to extend the shelf life of fresh and minimally processed fruits and vegetables: Quality and safety aspects. Microorganisms 2020, 8, 952. [Google Scholar] [CrossRef] [PubMed]
- Peltzer, M.A.; Salvay, A.G.; Delgado, J.F.; Wagner, J.R. Use of edible films and coatings for functional foods developments: A review. In Functional Foods: Sources, Health Effects and Future Perspectives; NOVA: New York, NY, USA, 2017; pp. 1–26. [Google Scholar]
- Soukoulis, C.; Behboudi-Jobbehdar, S.; Macnaughtan, W.; Parmenter, C.; Fisk, I.D. Stability of Lactobacillus rhamnosus GG incorporated in edible films: Impact of anionic biopolymers and whey protein concentrate. Food Hydrocoll. 2017, 70, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Marín, A.; Cháfer, M.; Atarés, L.; Chiralt, A.; Torres, R.; Usall, J.; Teixidó, N. Effect of different coating-forming agents on the efficacy of the biocontrol agent Candida sake CPA-1 for control of Botrytis cinerea on grapes. Biol. Control 2016, 96, 108–119. [Google Scholar] [CrossRef] [Green Version]
- Soukoulis, C.; Behboudi-Jobbehdar, S.; Yonekura, L.; Parmenter, C.; Fisk, I.D. Stability of Lactobacillus rhamnosus GG in prebiotic edible films. Food Chem. 2014, 159, 302–308. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Saavedra, J.I.Q.; Chiralt, A. Physical properties and antilisterial activity of bioactive edible films containing Lactobacillus plantarum. Food Hydrocoll. 2013, 33, 92–98. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Saavedra, J.I.Q.; Chiralt, A. Antilisterial and physical properties of biopolymer films containing lactic acid bacteria. Food Control 2014, 35, 200–206. [Google Scholar] [CrossRef]
- Racioppo, A.; Corbo, M.R.; Piccoli, C.; Sinigaglia, M.; Speranza, B.; Bevilacqua, A. Ultrasound attenuation of lactobacilli and bifidobacteria: Effect on some technological and probiotic properties. Int. J. Food Microbiol. 2017, 243, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Parra, J.; Mejía, C.M.; Villa, C.C. Development of a bioactive synbiotic edible film based on cassava starch, inulin, and Lactobacillus casei. Food Hydrocoll. 2020, 104, 105754. [Google Scholar] [CrossRef]
- Muley, A.B.; Singhal, R.S. Extension of postharvest shelf life of strawberries (Fragaria ananassa) using a coating of chitosan-whey protein isolate conjugate. Food Chem. 2020, 329, 127213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, W.; Sun, L.; Sadiq, F.A.; Yang, Y.; Gao, J.; Sang, Y. Preparation screening, production optimization and characterization of exopolysaccharides produced by Lactobacillus sanfranciscensis Ls-1001 isolated from Chinese traditional sourdough. Int. J. Biol. Macromol. 2019, 139, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Min, W.H.; Fang, X.B.; Wu, T.; Fang, L.; Liu, C.L.; Wang, J. Characterization and antioxidant activity of an acidic exopolysaccharide from Lactobacillus plantarum JLAU103. J. Biosci. Bioeng. 2019, 127, 758–766. [Google Scholar] [CrossRef] [PubMed]

| Probiotic Bacteria | Materials Used to Formulate Edible Coating/Films | Additives | Fruit/Vegetable | Reference |
|---|---|---|---|---|
| Lacticaseibacillus casei | Alginate | Glycerol (5 g/L); polysorbate 80 (1 g/L); linseed mucilage (0.3° Brix); FOS (15 g/L); calcium chloride solution (20 g/L) | Fresh-cut yacon | [20] |
| Lactobacillus acidophilus | Alginate solution | Glycerol (0.5 g); sunflower oil (0.075 g); Tween 80 (0.025 g); bivalent Ca2+ ion | Minimally processed carrot | [21] |
| Lactiplantibacillus plantarum | Alginate + chitosan | Citrate (0.2%, w/v), ascorbate (1% w/v), CaCl2 (0.5%, w/v) | Apple and melon pieces | [18] |
| Lacticaseibacillus rhamnosus | Alginate + prebiotics | Glycerol (15 g/kg); inulin andoligofructose (40 and 80 g/kg); CaCl2 (20 g/kg) | Fresh blueberry | [22] |
| L. casei | Whey protein isolate | Glycerol (5%, w/v) | Cherry tomato and Thompson grape | [23] |
| L. rhamnosus | Alginate + prebiotic | Glycerol (1%); inulin (non-detailed concentration); CaCl2 (2% w/v) | Minimally processed apple | [24] |
| L. plantarum | Carboxymethylcellulose | Glycerol 30% (w/w) | Strawberry | [25] |
| L. plantarum | Pregelatinized potato starch or sodium caseinate | Oleic acid (ratio 0.1:1) | Grape | [26] |
| L. acidophilus | Sodium alginate | Glycerol (0.75 g); sunflower oil (0.04 g), Tween 80 (0.05 g); coconut water (70%) | Minimally processed carrot | [27] |
| Bifidobacterium animalis subsp. lactis | Alginate + gelatin | CaCl2 (0.5%, w/v) | Apple pieces | [14] |
| Lacticaseibacillus paracasei | Corn starch + gelatin | Glycerol (1%, w/w); Gamma radiation (3.5 KGy) | Tomato | [6] |
| L. plantarum, Pediococcus pentosaceus | Cassava starch | Glycerol (1.5%, w/w); carboxymethylcellulose (0.2%, w/w) | Banana | [28] |
| L. rhamnosus, B.lactis | Alginate + prebiotics | Glycerol (1.5%, w/w); inulin (8%, w/w) and oligofructose (8%, w/w); CaCl2 (2%, w/v) | Fresh-cut apple | [29] |
| L. plantarum AF1, L. plantarum LU5, L. plantarum LP3 | Konjac glucomannan (gum) | Glycerol (25%, w/w) | Fresh-cut kiwi | [2] |
| L. rhamnosus | Gelatin + prebiotic | Glycerol (15%, w/w); inulin (2.5%, w/w) | Strawberry | [12] |
| Probiotic Bacteria | Materials and Concentrations Used to Formulate Edible Coating/Films | Initial Inoculum (log CFU/g or mL) | Final Viable Counts (log CFU/g or mL) | Storage Condition | Reference |
|---|---|---|---|---|---|
| Lacticaseibacilluscasei LC-01 | Alginate (20 g/L) | 8–9 | 8.0–8.7 | 5 °C, 15 days | [20] |
| Lactobacillus acidophilus La-14 | Alginate solution (1.75%, w/w) | 7.36 | 7.1 | 8 °C, 19 days | [21] |
| Lactipnatibacillus plantarum c19 | Alginate (2%, w/v) + chitosan (1%, w/v) | 6.8 | 4.5–5.3 (chitosan) and 6.7–7.3 (alginate) | 4 °C, 14 days | [18] |
| Lacticaseibacillusrhamnosus CECT8361 | Alginate (20 g/kg) + prebiotics | 7.1–7.6 | 5 (without prebiotic) and 6.2 (with prebiotic) | 5 °C, 21 days | [22] |
| L. casei 01 | Whey protein isolate (10%, w/v) | 7.8 | 5.7 | 25 °C, 28 days | [23] |
| L. rhamnosus B-445 | Alginate (2%, w/v) + prebiotic | 8.22–8.34 | 6.0–7.4 | 5 °C, 13 days | [24] |
| L. plantarum PTCC1058 | Carboxymethylcellulose (1%, w/v) | 6.52–8.90 | 5.3–8.4 | 4 °C, 15 days | [25] |
| L. plantarum | Pregelatinized potato starch (2%, w/v) or sodium caseinate (2%, w/v) | 7.7 | 4.1–5.2 (pregelatinized potato starch); 6.1–6.2 g (sodium caseinate) | 20 °C, 7 or 9 days | [26] |
| L. acidophilus LA3 | Sodium alginate (1.5%, w/w) | 9 | <4 (alginate/prebiotic); 6.3 (alginate/coconut water/prebiotic) | 8 °C, 21 days | [27] |
| Bifidobacterium animalis subsp. lactis DSM10140 | Alginate (2%, w/v) or gelatin (sucrose 0.5%—w/v, corn starch 0.08%—w/v, lemon juice 0.05%—v/v) | 8 | 8.0–6.8 (alginate coated, 8 °C) * | 4 and 8 °C, 10 days | [14] |
| Lacticaseibacillus paracasei | Corn starch (4 g/mL) + gelatin (1 g/mL) | NP | NP | 5 °C, 14 days | [6] |
| L. plantarum and Pediococcus pentosaceus | Cassava starch (4%, w/w) | ~8 and 9 | ~7 and 8 | 30 °C, 48 h (drying) | [28] |
| L.rhamnosus CECT 8361, B. lactis CECT 8145 | Alginate (2%, w/w) + prebiotics | 11.7 | 9.1–9.5 | 5 °C, 8 days | [29] |
| L. plantarum AF1, L. plantarum LU5, L. plantarum LP3 | Konjac glucomannan (6%, w/w) | 9.4 | 6.4–7.1 | 4 °C, 5 days | [2] |
| L. rhamnosus HN001 | Gelatin (5%, w/w) + prebiotic | 11 | 7.0–7.4 (with prebiotic compounds) | 4 °C, 16 days | [12] |
| Probiotics | Target Microorganism/Microbial Group | Fruit/Vegetables | Main Effects of Probiotic Coating | Reference |
|---|---|---|---|---|
| Lacticaseibacillus casei | NP | Fresh-cut yacon | NP | [20] |
| Lactobacillus acidophilus | Aerobic mesophilic bacteira; molds and yeasts | Minimally processed carrot | Inhibited the fungal growth during the 19 days of storage. Uncoated fruit had higher levels of mold and yeast contamination at the end of the storage period. | [21] |
| Lactiplantibacillus plantarum | Molds and yeasts and psychrotrophic bacteria | Apple and melon pieces | Counts of molds, yeasts and psychrotrophic bacteria were below the limit of detection during the storage. | [18] |
| Lacticaseibacillus rhamnosus | Native microbiota; Listeria innocua and Escherichia coli O157:H7 | Fresh-blueberry | Counts of native microbiota remained at safe levels during refrigeration storage. Reduction of counts of L. innocua up to 1.7 log units. | [22] |
| L. casei | NP | Cherry tomatoes and grape | NP | [23] |
| L. rhamnosus | Mesophilic bacteria and molds and yeasts | Minimally processed apple | Counts of mesophilic bacteria, molds and yeast were reduced, extending the shelf life of fresh-cut apples. | [24] |
| L. plantarum | Molds and yeasts | Strawberry | Reduction of mold and yeast counts on strawberries. Inverse correlation between the number of viable probiotic cells and population of molds and yeasts, indicating a dose-dependent effect. | [25] |
| L. plantarum | Botrytis cinerea | Grape | Reduction of incidence and severity of B. cinerea infection. Potato starch-based formulation without oleic acid reduced the B. cinerea incidence more than when applied in sodium caseinate formulation or in water. | [26] |
| L. acidophilus | Thermotolerant coliforms, molds and yeasts, Salmonella spp. | Minimally processed carrot | Carrot submitted to the different treatments had absence of thermotolerant coliforms, Salmonella spp. and molds and yeasts during storage. | [27] |
| Bifidobacterium animalis subsp. lactis | NP | Apple pieces | NP | [14] |
| Lacticaseibacillus paracasei | Native microbiota | Tomato | Coated tomato had the lowest percentage of rot and bacterial counts at the end of the storage period, which were attributed to the effects of gamma irradiation increasing the antimicrobial activity of irradiated lactic acid bacteria supernatant. | [6] |
| L. plantarum, Pediococcus pentosaceus | NP | Banana | NP | [28] |
| L. rhamnosus, B.lactis | E. coli O157:H7; L. innocua; molds and yeasts | Fresh-cut apple | Maintenance of the microbiological quality of coated apples. | [29] |
| L. plantarum AF1, L. plantarum LU5, L. plantarum LP3 | Molds and yeasts | Fresh-cut kiwi | Coated kiwi slices had reduced mold and yeast counts. | [2] |
| L. rhamnosus | Molds and yeasts; aerobic mesophilic bacteria | Strawberry | Coated strawberries had reduced counts of mesophilic bacteria and molds and yeasts. | [12] |
| Probiotic Bacteria | Coating/Film | Fruit/Vegetable | Main Effects Related to Physicochemical Parameters | Main Effects Related to Sensory Parameters | Reference |
|---|---|---|---|---|---|
| Lacticaseibacillus casei LC-01 | Alginate, linseed mucilage, fructooligosaccharides | Fresh-cut yacon | Coated yacon had reduced weight loss and maintained the acidity and soluble solids contents during refrigeration storage. | Coated fruit had decreased darkening. | [20] |
| Lactobacillus acidophilus | Alginate solution | Minimally processed carrot | Coated carrot had reduced metabolism, with less variation in acidity, and maintained the moisture content during refrigeration storage. | Coated fruit had decreased color change (white surface discoloration). | [21] |
| Lactiplantibacillus plantarum | Alginate powder or chitosan | Apple and melon pieces | Alginate coating caused higher probiotic survival on fruit, and decreased the negative effects of the probiotic-loaded coatings on color and pH of fruit during refrigeration storage. | NP | [18] |
| Lacticaseibacillus rhamnosus | Alginate, prebiotic | Fresh blueberry | Coating had no effects on instrumental firmness and color of fruit. | Coated fruit had satisfactory visual quality, odor and flavor, being sensorially acceptable up to day 14 of refrigeration storage. | [22] |
| L. casei | Whey protein isolate | Cherry tomato, Thompson grape | Coated grape and tomato had delayed ripening evolution. High probiotic viable counts on coated fruit were found for up to 14 days of room temperature storage. | NP | [23] |
| L. rhamnosus | Alginate, prebiotic | Minimally processed apple | Coated apple maintained the moisture content, total soluble solids, firmness, ascorbic acid, pH and titratable acidity during refrigeration storage. | Color, odor, taste and texture characteristics of coated fruit were maintained up to 13 days of storage. | [24] |
| L. plantarum | Carboxymethylcellulose | Strawberries | Coating had positive effects on the physicochemical parameters of strawberries, reducing the weight loss and slowing down the deterioration rate of ascorbic acid and phenolic compounds during refrigeration storage. | Sensory characteristics of coated fruit were not affected, which were acceptable in terms of color, flavor, taste, texture and overall acceptability during storage. | [25] |
| L. plantarum | Pregelatinized potato starch or sodium caseinate | Grape | Coatings had little effect on weight, color, firmness and soluble solids contents of grapes during room temperature storage. | NP | [26] |
| L. acidophilus | Sodium alginate | Minimally processed carrot | NP | Improvement of the sensory attributes of coated fruit, particularly of color, appearance and texture. | [27] |
| Bifidobacterium animalis subsp. lactis | Alginate, gelatin | Apple chips | Addition of isolated probiotic caused worsening of color of apple chips, with an increase in browning index. Probiotic-loaded coating counteracted this negative effect. | Coated apple pieces had higher sensory scores and lower browning index after 10 days of refrigeration storage. | [14] |
| Lacticaseibacillus paracasei | Corn starch, gelatin | Tomato | Coated tomato had decreased weight loss and decay percentage, and higher ascorbic acid, lycopene, total sugars and total phenolic contents. | NP | [6] |
| L. plantarum, Pediococcus pentosaceus | Cassava starch | Banana | Coated banana had prolonged shelf life and reduced black spot development for up to 7 days of storage. | NP | [28] |
| L. rhamnosus, B. lactis | Alginate, prebiotic | Fresh-cut apple | Coated apple maintained the total phenolic contents and antioxidant capacities during refrigeration storage. | Apple coated with prebiotic and B. lactis remained sensorially acceptable up to 8 days of storage | [29] |
| L. plantarum | Konjac glucomannan | Fresh-cut kiwi | Coated kiwi had decreased decay and color changes, higher total phenolic content and antioxidant capacities, and maintained chlorophyll and ascorbic acid contents during refrigeration storage. | Probiotic treatments positively influenced the overall acceptability of fruit, while uncoated fruit were rejected. | [2] |
| L. rhamnosus | Gelatin, prebiotic | Strawberry | Coated strawberry had decreased weight loss and preserved the total phenolic contents and antioxidant capacity during refrigeration storage. | NP | [12] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, K.Á.R.; Fernandes, K.F.D.; de Souza, E.L. Current Advances on the Development and Application of Probiotic-Loaded Edible Films and Coatings for the Bioprotection of Fresh and Minimally Processed Fruit and Vegetables. Foods 2021, 10, 2207. https://doi.org/10.3390/foods10092207
de Oliveira KÁR, Fernandes KFD, de Souza EL. Current Advances on the Development and Application of Probiotic-Loaded Edible Films and Coatings for the Bioprotection of Fresh and Minimally Processed Fruit and Vegetables. Foods. 2021; 10(9):2207. https://doi.org/10.3390/foods10092207
Chicago/Turabian Stylede Oliveira, Kataryne Árabe Rimá, Karina Felix Dias Fernandes, and Evandro Leite de Souza. 2021. "Current Advances on the Development and Application of Probiotic-Loaded Edible Films and Coatings for the Bioprotection of Fresh and Minimally Processed Fruit and Vegetables" Foods 10, no. 9: 2207. https://doi.org/10.3390/foods10092207
APA Stylede Oliveira, K. Á. R., Fernandes, K. F. D., & de Souza, E. L. (2021). Current Advances on the Development and Application of Probiotic-Loaded Edible Films and Coatings for the Bioprotection of Fresh and Minimally Processed Fruit and Vegetables. Foods, 10(9), 2207. https://doi.org/10.3390/foods10092207